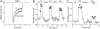Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex - PubMed (original) (raw)
Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex
Guojun Chen et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Interactions between dopamine and N-methyl-D-aspartate receptors (NMDARs) in prefrontal cortex (PFC) and other brain regions are believed to play an important role in normal mental function and neuropsychiatric disorders. In this study, we examined the regulation of NMDAR currents by the dopamine D1 receptor in PFC pyramidal neurons. Application of the D1 receptor agonist SKF81297 caused a prominent increase of the steady-state NMDA-evoked current in acutely isolated PFC pyramidal neurons. The D1 effect on NMDARs was independent of protein kinase A or protein phosphatase 1, but was abolished by incubation of neurons in Ca2+-free medium. Intracellular application of the Ca2+ chelator, calmodulin, or calmodulin inhibitors largely prevented the D1 modulation of NMDAR currents. Moreover, inhibiting PKC activity or disrupting PKC association with its anchoring protein also significantly reduced the D1 effect on NMDAR currents. This upregulation of NMDAR activity by dopamine D1 receptors and the previous finding on up-regulation of dopamine D1 receptors by NMDAR activation provide a cellular mechanism for the reciprocal interactions between D1 and NMDARs. These interactions may play an important role in modulating synaptic plasticity and thus in cognitive and emotional processes.
Figures
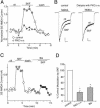
Fig. 1.
Activation of D1 receptors reversibly enhanced NMDAR currents in acutely dissociated PFC pyramidal neurons. (A) Current traces taken from a representative neuron showing the effect of SKF81297 (10 μM) on NMDA (500 μM)-evoked currents. (B) Plot of the steady-state NMDAR current (_I_ss) as a function of time and agonist application. (C) Plot of _I_ss showing that the selective D1 antagonist SCH23390 (SCH) (10 μM) largely blocked the effect of SKF81297 (SKF).
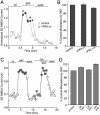
Fig. 2.
The effect of SKF81297 (SKF) on NMDAR currents was independent of PKA/PP1. (A) Plot of _I_ss showing that dialysis with the PKA inhibitory peptide PKI6–22 (20 μM) did not prevent the SKF81297-induced potentiation of NMDAR currents. (B) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in the absence (n = 16) or presence of PKI6–22 (n = 15), or the membrane-permeable myristoylated PKA inhibitor PKI14–22 (0.2 μM, n = 10). (C) Plot of _I_ss showing that inhibiting PP1 activity with OA (0.1 μM) failed to affect the SKF81297-induced potentiation of NMDAR currents. (D) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in the absence (n = 34) or presence of OA (external application: 0.1 μM, n = 8; internal application: 1 μM, n = 23) or the PP1-anchoring inhibitory peptide Gm (20 μM, n = 20).
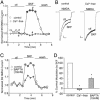
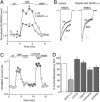
Fig. 3.
The effect of SKF81297 (SKF) on NMDAR currents depended on Ca2+. (A) Plot of _I_ss as a function of time and agonist application in neurons perfused in a Ca2+-free solution (10 μM EGTA added) or in the normal external solution (containing 1 mM Ca2+). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of _I_ss as a function of time and agonist application in neurons dialyzed with the high BAPTA internal solution (10 mM) or the normal internal solution (containing 0.5 mM BAPTA). (D) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in normal conditions (n = 20), in the Ca2+-free solution (n = 7), or in neurons dialyzed with high BAPTA (n = 15). *, P <0.001, ANOVA.
Fig. 4.
The effect of SKF81297 (SKF) on NMDAR currents depended on CaM. (A) Plot of _I_ss as a function of time and agonist application in neurons loaded with or without CaM (10 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of _I_ss as a function of time and agonist application in neurons dialyzed with or without the CaM antagonist CDZ (20 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in normal conditions (n = 10) or in neurons dialyzed with CaM (n = 7), CDZ (n = 14) or the CaM inhibitory peptide MLCK peptide (MLCKP) (n = 12), or in the presence of the CaM-dependent protein kinase II inhibitor KN-93 (10 μM, n = 14) or the calcineurin inhibitor CsA (50 μM, n = 5). *, P <0.001, ANOVA.
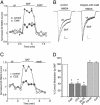
Fig. 5.
The effect of SKF81297 (SKF) on NMDAR currents was attenuated by inhibiting PKC. (A) Plot of _I_ss as a function of time and agonist application in neurons loaded with or without the PKC inhibitory peptide PKC19–36 (4 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of _I_ss showing that the enhancing effect of SKF81297 was largely diminished in the presence of the PKC inhibitor bisindolylmaleimide (Bis, 1 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in normal conditions (n = 11) or in neurons dialyzed with PKC19–36 (n = 17), or in the presence of bisindolylmaleimide (Bis, n = 21). *, P <0.001, ANOVA.
Fig. 6.
The effect of SKF81297 (SKF) on NMDAR currents was reduced by disrupting the PKC/AKAP interaction but was not affected by inhibiting phospholipase C (PLC) or other signaling molecules. (A) Plot of _I_ss as a function of time and agonist application in neurons loaded with or without the peptide AKAP31–52 (40 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of _I_ss showing that the enhancing effect of SKF81297 was intact in the presence of the PLC inhibitor U73122 (1 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of _I_ss by SKF81297 in normal conditions (n = 10) or in neurons dialyzed with AKAP31–52 (n = 15), or in the presence of U73122 (n = 4), the inositol-1-4-5-triphosphate receptor antagonist heparin (2 mg/ml, n = 4), the phosphoinositide 3-kinase inhibitor wortmannin (3 μM, n = 12), or the protein tyrosine kinase inhibitor genistein (100 μM, n = 16). *, P <0.001, ANOVA.
Similar articles
- Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex.
Wang X, Zhong P, Gu Z, Yan Z. Wang X, et al. J Neurosci. 2003 Oct 29;23(30):9852-61. doi: 10.1523/JNEUROSCI.23-30-09852.2003. J Neurosci. 2003. PMID: 14586014 Free PMC article. - Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala.
Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Pickel VM, et al. Neuroscience. 2006 Oct 27;142(3):671-90. doi: 10.1016/j.neuroscience.2006.06.059. Epub 2006 Aug 14. Neuroscience. 2006. PMID: 16905271 - Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex.
Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Tyszkiewicz JP, et al. J Physiol. 2004 Feb 1;554(Pt 3):765-77. doi: 10.1113/jphysiol.2003.056812. Epub 2003 Nov 28. J Physiol. 2004. PMID: 14645456 Free PMC article. - Where do you think you are going? The NMDA-D1 receptor trap.
Cepeda C, Levine MS. Cepeda C, et al. Sci STKE. 2006 May 2;2006(333):pe20. doi: 10.1126/stke.3332006pe20. Sci STKE. 2006. PMID: 16670371 Review.
Cited by
- Effect of dopaminergic D1 receptors on plasticity is dependent of serotoninergic 5-HT1A receptors in L5-pyramidal neurons of the prefrontal cortex.
Meunier CN, Callebert J, Cancela JM, Fossier P. Meunier CN, et al. PLoS One. 2015 Mar 16;10(3):e0120286. doi: 10.1371/journal.pone.0120286. eCollection 2015. PLoS One. 2015. PMID: 25775449 Free PMC article. - Cocaine withdrawal and neuro-adaptations in ion channel function.
Hu XT. Hu XT. Mol Neurobiol. 2007 Feb;35(1):95-112. doi: 10.1007/BF02700626. Mol Neurobiol. 2007. PMID: 17519508 Review. - Cytosolic sulfotransferase 1A3 is induced by dopamine and protects neuronal cells from dopamine toxicity: role of D1 receptor-N-methyl-D-aspartate receptor coupling.
Sidharthan NP, Minchin RF, Butcher NJ. Sidharthan NP, et al. J Biol Chem. 2013 Nov 29;288(48):34364-74. doi: 10.1074/jbc.M113.493239. Epub 2013 Oct 17. J Biol Chem. 2013. PMID: 24136195 Free PMC article. - Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model.
Lim CS, Hoang ET, Viar KE, Stornetta RL, Scott MM, Zhu JJ. Lim CS, et al. Genes Dev. 2014 Feb 1;28(3):273-89. doi: 10.1101/gad.232470.113. Genes Dev. 2014. PMID: 24493647 Free PMC article. - Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons.
Gao C, Wolf ME. Gao C, et al. J Neurochem. 2008 Sep;106(6):2489-501. doi: 10.1111/j.1471-4159.2008.05597.x. Epub 2008 Jul 30. J Neurochem. 2008. PMID: 18673451 Free PMC article.
References
- Goldman-Rakic, P. S. (1995) Neuron 14, 477-485. - PubMed
- Andreasen, N. C., O'Leary, D. S., Flaum, M., Nopoulos, P., Watkins, G. L., Boles Ponto, L. L. & Hichwa, R. D. (1997) Lancet 349, 1730-1734. - PubMed
- Lewis, D. A. & Lieberman, J. A. (2000) Neuron 28, 325-334. - PubMed
- Goldman-Rakic, P. S. (1994) J. Neuropsychiatry Clin. Neurosci. 6, 348-357. - PubMed
- Carlsson, A., Waters, N., Holm-Waters, S., Tedroff, J., Nilsson, M. & Carlsson, M. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 237-260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 MH040899/MH/NIMH NIH HHS/United States
- MH-63128/MH/NIMH NIH HHS/United States
- MH-40899/MH/NIMH NIH HHS/United States
- AG-21923/AG/NIA NIH HHS/United States
- P01 DA010044/DA/NIDA NIH HHS/United States
- R01 AG021923/AG/NIA NIH HHS/United States
- DA-10044/DA/NIDA NIH HHS/United States
- R01 MH063128/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous