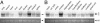Identification of a developmentally regulated striatum-enriched zinc-finger gene, Nolz-1, in the mammalian brain - PubMed (original) (raw)
. 2004 Feb 24;101(8):2613-8.
doi: 10.1073/pnas.0308645100.
Chi-Wei Tsai, Hsiao-Fang Wang, Hsiu-Chao Tsai, Huei-Ying Chen, Ting-Fen Tsai, Hiroshi Takahashi, Hui-Yun Li, Ming-Ji Fann, Chu-Wen Yang, Yoshihide Hayashizaki, Tetsuichiro Saito, Fu-Chin Liu
Affiliations
- PMID: 14983057
- PMCID: PMC356998
- DOI: 10.1073/pnas.0308645100
Identification of a developmentally regulated striatum-enriched zinc-finger gene, Nolz-1, in the mammalian brain
Chiung-Wen Chang et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Neural information processed through the striatum of the basal ganglia is crucial for sensorimotor and psychomotor functions. Genes that are highly expressed in the striatum during development may be involved in neural development and plasticity in the striatum. We report in the present study the identification of a previously uncharacterized mammalian member of the nocA/elB/tlp-1 family, Nolz-1, that is preferentially expressed at high levels in the developing striatum. Nolz-1 mRNA was expressed as soon as striatal anlage began to form at embryonic day 13 in the rat. Nolz-1 mRNA was predominantly expressed in the lateral ganglionic eminence (striatal primordium) and was nearly absent in the adjacent structures of the medial ganglionic eminence and the cerebral cortex. Moreover, Nolz-1 was highly expressed in the subventricular zone of the lateral ganglionic eminence and was colocalized with the early neuronal differentiation markers of TuJ1 and Isl1 and the projection neuron marker of DARPP-32, suggesting that Nolz-1 was expressed in differentiating progenitors of striatal projection neurons. A time course study showed that Nolz-1 mRNA was developmentally regulated, as its expression was down-regulated postnatally with low levels remaining in the ventral striatum at adulthood. As the tagged Nolz-1 protein was localized in the nucleus, Nolz-1 may function as transcriptional regulator. In a model system for neural differentiation, Nolz-1 mRNA was dramatically induced on neural induction of P19 embryonal carcinoma cells by retinoic acid, suggesting that Nolz-1 activation may be involved in neural differentiation. Our study suggests that Nolz-1 is preferentially expressed in differentiating striatal progenitors and may be engaged in the genetic program for controlling striatal development.
Figures
Fig. 1.
(A) The deduced amino acid sequences of mNolz-1. The following domains are marked: glycine (G)-rich domains (17–56 and 146–328; underlined); serine (S)-rich domain (320–340; italic and bold); alanine (A)-rich domains (398–496 and 570–576; italic); Groucho binding sequence (bold); C2H2 zinc-finger motif (bold and underlined); Zn2+ binding motif [two cysteine (C) and two histidine (H); bold, italic, and underlined]; amino acids that differ from hNolz-1 (shading). (B and C) Amino acid sequence alignments of the nocA/elB family in amino-terminal (B) and carboxyl-terminal (C) domains showing high homology among the family members. The identical residues are indicated by black and shaded boxes. The zinc-finger motifs are in italic, and the consensus residues are shown in bold below (C). The sequences of mNolz-1 shown in B and C are identical for rNolz-1 and hNolz-1.
Fig. 2.
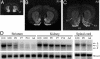
Northern blots of Nolz-1 transcripts in E20 rat embryo. Shown is expression of Nolz-1 in different organ tissues of E20 rat embryo (A) and in different regions of E20 rat brain (B). Note that a doublet of transcripts consisting of the E and C forms are detected in the striatum.
Fig. 3.
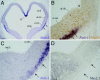
Expression of Nolz-1 mRNA in E13 rat telencephalon. (A) Nolz-l mRNA (double arrows) is expressed in the lateral part of ventral LGE (vLGE). (B) Nolz-1 is not expressed in the dorsal LGE (dLGE) containing Mash1-positive nuclei (arrow), nor is it expressed in the Nkx2.1-positive MGE (D) or the cortical primordium (CTX). The arrowhead in B indicates the border between the dLGE and CTX. The sections in C and D are adjacent sections, and the two arrows point to the _Nolz-1_-positive streak slightly extending into the Nkx2.1-poor region in the MGE. LT, lamina terminalis. (Scale bars: A, 200 μm; D for B_–_D, 50 μm.)
Fig. 4.
Expression of Nolz-1 mRNA in E15 rat telencephalon. (A_–_C) Nolz-1 mRNA is primarily expressed in the LGE at rostral (A), middle (B), and caudal (C) levels. (D) Double labeling of Nolz-1 mRNA and MASH1 protein. Nolz-1 is highly expressed in the SVZ but is not expressed in the MASH1-positive ventricular zone (VZ, brown nuclei). Nolz-1 expression is decreased in the mantle zone (MZ). (E and F) Adjacent sections showing spatial correspondence of Nolz-1 and Raldh-3 mRNA expression in the SVZ of LGE. (G) Coexpression of Nolz-1 mRNA (purple cytosolic ring) and Isl1 protein (brown nucleus) in early differentiating striatal cells (arrow) in the SVZ of LGE. (H) Double labeling of Nolz-1 mRNA (purple cytosolic ring) and DARPP-32 protein (brown nucleus and cytosol) in E20 striatal neurons (arrow, dark brown cytosol). (I and J) Coexpression of Nolz-1 mRNA (I) and TuJ1 protein (J) in differentiating neurons (arrows) in SVZ of LGE. (K_–_N) Nuclear localization of FLAG-tagged Nolz-1 protein in HEK 293T cells (K_–_M, arrowheads) and mouse E13.5 forebrain (N). (K) FLAG immunostaining. (L) Hoechst 33258 nuclear staining. (M) Merged images of K and L.(N) A cotransfected GFP-positive cell with neuronal morphology in the brain shows nuclear localization of tagged Nolz-1 protein (red nucleus). (Scale bars: A for A_–_C, 200 μm; D for D_–_F, 200 μm; G for G_–_J, 2 μm; K for K_–_M, 5 μm; N, 5 μm.)
Fig. 5.
Expression of Nolz-1 mRNA in E20 rat forebrain. (A) Nolz-1 mRNA is highly expressed in the SVZ of the developing striatum (ST) except the dorsal cap, which lacks Nolz-1 (asterisk). The bracketed region is shown at high magnification in Inset.(B) Film autoradiogram of a parasagittal section showing enriched expression of Nolz-1 mRNA in the striatum of E20 rat brain with 35S-labeled probes. CTX, cortex; HB, hindbrain; OB, olfactory bulb; OT, olfactory tubercle; SC, superior colliculus. (Scale bars: A, 200 μm; B, 500 μm.)
Fig. 6.
Developmental regulation of Nolz-1 mRNA in the striatum during development. Expression of Nolz-1 mRNA is gradually decreased from postnatal day 0 (A) and postnatal day 14 (B) to adult (Ad) striatum (ST; C) as assayed by in situ hybridization with 35S-labeled probes. The Nolz-1 expression remains in the nucleus accumbens (NA) and olfactory tubercle (OT) at adulthood. (D) Dynamic regulation of different Nolz-1 transcripts in the striatum during development. A triplet of transcripts consisting of the E, C, and P forms are present only in the postnatal day 0 striatum. No such complex regulation of Nolz-1 transcripts is detected in the development of the kidney and the spinal cord where Nolz-1 expression is also abundant. (Scale bar: A for A_–_C, 1 mm.)
Fig. 7.
Regulation of Nolz-1 in P19 cells and the LGE culture. (A) A doublet of Nolz-1 transcripts similar to the E and C forms found in E15, E18, and postnatal day 0 striatum (ST) are induced in aggregated (agg) P19 cells with RA treatment (RAagg). Little or no Nolz-1 induction is found with vehicle (Vehagg) or DMSO (DMSOagg) treatments, or without any treatment (Noneagg). (B) After the cell aggregates are dissociated for plating and cultured without RA (Noneplating), only the C form remains in the dissociated cells. (C) The pGL3-mNolz-1-P1.4 luciferase reporter gene vector was transfected into E16 rat LGE explant culture. All-trans RA increases the luciferase activity by 1.3-fold. This inductive effect is abolished when the RARE-containing region is deleted from the construct (pGL3-mNolz-1-dRARE). **, P < 0.01, Student's t test. A0–A4, 0–4 days after cell aggregation; P1–P5, 1–5 days after dissociating cell aggregates for dissociated cells culture.
Similar articles
- Cell type-selective expression of the zinc finger-containing gene Nolz-1/Zfp503 in the developing mouse striatum.
Ko HA, Chen SY, Chen HY, Hao HJ, Liu FC. Ko HA, et al. Neurosci Lett. 2013 Aug 26;548:44-9. doi: 10.1016/j.neulet.2013.05.020. Epub 2013 May 16. Neurosci Lett. 2013. PMID: 23684982 - Ectopic expression of nolz-1 in neural progenitors promotes cell cycle exit/premature neuronal differentiation accompanying with abnormal apoptosis in the developing mouse telencephalon.
Chang SL, Chen SY, Huang HH, Ko HA, Liu PT, Liu YC, Chen PH, Liu FC. Chang SL, et al. PLoS One. 2013 Sep 20;8(9):e74975. doi: 10.1371/journal.pone.0074975. eCollection 2013. PLoS One. 2013. PMID: 24073229 Free PMC article. - Region- and cell type-selective expression of the evolutionarily conserved Nolz-1/zfp503 gene in the developing mouse hindbrain.
Chang SL, Yan YT, Shi YL, Liu YC, Takahashi H, Liu FC. Chang SL, et al. Gene Expr Patterns. 2011 Dec;11(8):525-32. doi: 10.1016/j.gep.2011.09.003. Epub 2011 Sep 16. Gene Expr Patterns. 2011. PMID: 21945624 - Regulation of multiple dopamine signal transduction molecules by retinoids in the developing striatum.
Wang HF, Liu FC. Wang HF, et al. Neuroscience. 2005;134(1):97-105. doi: 10.1016/j.neuroscience.2005.04.008. Neuroscience. 2005. PMID: 15939542 - From Progenitors to Progeny: Shaping Striatal Circuit Development and Function.
Knowles R, Dehorter N, Ellender T. Knowles R, et al. J Neurosci. 2021 Nov 17;41(46):9483-9502. doi: 10.1523/JNEUROSCI.0620-21.2021. J Neurosci. 2021. PMID: 34789560 Free PMC article. Review.
Cited by
- A new paradigm for generating high-quality cardiac pacemaker cells from mouse pluripotent stem cells.
Lin Z, Lin B, Hang C, Lu R, Xiong H, Liu J, Wang S, Gong Z, Zhang M, Li D, Fang G, Ding J, Su X, Guo H, Shi D, Xie D, Liu Y, Liang D, Yang J, Chen YH. Lin Z, et al. Signal Transduct Target Ther. 2024 Sep 6;9(1):230. doi: 10.1038/s41392-024-01942-w. Signal Transduct Target Ther. 2024. PMID: 39237509 Free PMC article. - Insights into the pleiotropic roles of ZNF703 in cancer.
Wang S, Liu R. Wang S, et al. Heliyon. 2023 Sep 14;9(9):e20140. doi: 10.1016/j.heliyon.2023.e20140. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810156 Free PMC article. Review. - Zfp503/Nlz2 Is Required for RPE Differentiation and Optic Fissure Closure.
Boobalan E, Thompson AH, Alur RP, McGaughey DM, Dong L, Shih G, Vieta-Ferrer ER, Onojafe IF, Kalaskar VK, Arno G, Lotery AJ, Guan B, Bender C, Memon O, Brinster L, Soleilhavoup C, Panman L, Badea TC, Minella A, Lopez AJ, Thomasy SM, Moshiri A, Blain D, Hufnagel RB, Cogliati T, Bharti K, Brooks BP. Boobalan E, et al. Invest Ophthalmol Vis Sci. 2022 Nov 1;63(12):5. doi: 10.1167/iovs.63.12.5. Invest Ophthalmol Vis Sci. 2022. PMID: 36326727 Free PMC article. - The transcription factor Zfp503 promotes the D1 MSN identity and represses the D2 MSN identity.
Shang Z, Yang L, Wang Z, Tian Y, Gao Y, Su Z, Guo R, Li W, Liu G, Li X, Yang Z, Li Z, Zhang Z. Shang Z, et al. Front Cell Dev Biol. 2022 Aug 23;10:948331. doi: 10.3389/fcell.2022.948331. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36081908 Free PMC article. - Generalized and social anxiety disorder interactomes show distinctive overlaps with striosome and matrix interactomes.
Karunakaran KB, Amemori S, Balakrishnan N, Ganapathiraju MK, Amemori KI. Karunakaran KB, et al. Sci Rep. 2021 Sep 15;11(1):18392. doi: 10.1038/s41598-021-97418-w. Sci Rep. 2021. PMID: 34526518 Free PMC article.
References
- Graybiel, A. M. (1995) Curr. Opin. Neurobiol. 5, 733-741. - PubMed
- Wichmann, T. & DeLong, M. R. (1996) Curr. Opin. Neurobiol. 6, 751-758. - PubMed
- Graybiel, A. M. & Rauch, S. L. (2000) Neuron 28, 343-347. - PubMed
- Porteus, M. H., Bulfone, A., Ciaranello, R. D. & Rubenstein, J. L. R. (1991) Neuron 7, 221-229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials