S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity - PubMed (original) (raw)
S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity
Kandasam Ravi et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Endothelial nitric oxide synthase (eNOS) is active only as a homodimer. Recent data has demonstrated that exogenous NO can act as an inhibitor of eNOS activity both in intact animals and vascular endothelial cells. However, the exact mechanism by which NO exerts its inhibitory action is unclear. Our initial experiments in bovine aortic endothelial cells indicated that exogenous NO decreased NOS activity with an associated decrease in eNOS dimer levels. We then undertook a series of studies to investigate the mechanism of dimer disruption. Exposure of purified human eNOS protein to NO donors or calcium-mediated activation of the enzyme resulted in a shift in eNOS from a predominantly dimeric to a predominantly monomeric enzyme. Further studies indicated that endogenous NOS activity or NO exposure caused S-nitrosylation of eNOS and that the presence of the thioredoxin and thioredoxin reductase system could significantly protect eNOS dimer levels and prevent the resultant monomerization and loss of activity. Further, exogenous NO treatment caused zinc tetrathiolate cluster destruction at the dimer interface. To further determine whether S-nitrosylation within this region could explain the effect of NO on eNOS, we purified a C99A eNOS mutant enzyme lacking the tetrathiolate cluster and analyzed its oligomeric state. This enzyme was predominantly monomeric, implicating a role for the tetrathiolate cluster in dimer maintenance and stability. Therefore, this study links the inhibitory action of NO with the destruction of zinc tetrathiolate cluster at the dimeric interface through S-nitrosylation of the cysteine residues.
Figures
Fig. 1.
Effect of exogenous NO on eNOS in BAECs. BAECs were treated (+) or untreated (-) with SPERNO (100 μM) for 1 h, then eNOS dimer levels were determined. Protein extracts (20 μg) were subjected to LT-PAGE and Western blot analyses. A representative Western blot is shown from n = 4.
Fig. 2.
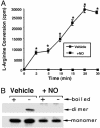
NO-mediated inactivation of purified human eNOS. (A) Purified human eNOS protein (2 μg) was tested for its ability to metabolize [2,3,4,5-3H]-
l
arginine in the presence (+NO) or absence (vehicle) of the NO donor SPERNO (100 μM). The presence of the NO donor results in the total inhibition of the recombinant eNOS protein. *, P < 0.05 vs. SPERNO. (B) Purified human eNOS (400 ng) was exposed (30 min at 37°C) to the NO donor SPERNO (100 μM, + NO), was subjected to LT-PAGE and Western blot analyses, and was compared with eNOS protein exposed only to sodium phosphate buffer, pH 7.4 (vehicle). +, eNOS protein exposed to 95°C for 5 min before loading to monomerize all eNOS protein; -, eNOS protein not boiled before loading. A representative Western blot is shown from n = 4.
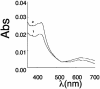
Fig. 3.
Light absorbance spectra of purified eNOS before and after NO treatment. Curve 1, eNOS (10 μM) in 40 mM EPPS with10% glycerol and 0.1 mM DTT. Initially, a mixture of ferric and ferrous peaks is observed, however, after 2 min, there is complete oxidation to ferric heme with absorbance maxima at ≈420. Curve 2, spectra of the above eNOS mixture with 100 μM SPERNO incubated at 37°C for 30 min showing no loss of heme.
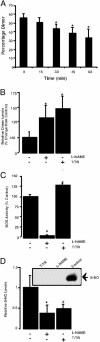
Fig. 4.
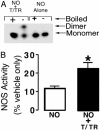
Calcium-dependent activation of eNOS produces a decrease in dimer levels and increases S-nitrosylation. (A) Densitometric analysis of LT-PAGE of activated eNOS (2 μg) showing time-dependent decreases in dimer levels. Western blot analysis of activated eNOS in the presence of all of the cofactors and excess substrate (0–60 min). Activation of eNOS results in degradation of dimeric. (n = 3.) *, < 0.05 vs. T = 0 min. (B) The decrease in NOS dimer levels is reduced by the NOS inhibitor
l
-NAME or the disulfide reductase system (T/TR). (n = 5.) *, < 0.05 vs. control. (C) The presence of the disulfide reeducates system (T/TR) increases the activity of the eNOS enzyme. (n = 4.) *, < 0.05 vs. control. (D) The calcium-dependent activation of eNOS increases the S-nitrosylation of the enzyme that is inhibited by
l
-NAME or the disulfide reeducates system (T/TR). (Inset) A representative image from n = 3. *, P < 0.05 vs. control.
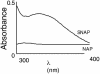
Fig. 5.
NO induces S-nitrosylation of recombinant human eNOS. DTT-free human eNOS protein (500 μg) was exposed to _S_-nitroso-acetyl penicillamine (SNAP) (1 mM) for 1 h at 37°C in the dark. The nitrosylating reagent was removed by using a spin column, and a spectral scan was carried out in the range of 300–400 nm. As a control, the human eNOS protein was treated with the non-NO releasing agent, _N_-acetyl penicillamine (NAP) (1 mM).
Fig. 6.
The presence of the disulfide reductase system protects the human eNOS dimer from the effects of NO. (A) Purified human eNOS (400 ng) was exposed, for 30 min at 37°C, to the NO donor SPERNO (100 μM), either alone (NO alone) or after a preincubation with thioredoxin (5 μM) and thioredoxin reductase (6.4 μM) in the presence of 0.2 mM NADPH (NO + T/TR). Each fraction was then subjected to LT-PAGE and Western blot analyses. +, eNOS protein exposed to 95°C for 5 min before loading to monomerize all eNOS protein; -, eNOS protein not boiled before loading. (B). Assays were carried out with purified eNOS (2 μg) to determine the effect of preincubation with thioredoxin (5 μM), thioredoxin reductase (6.4 μM), and 0.2 mM NADPH on the ability of eNOS to metabolize [2,3,4,5-3H]-
l
-arginine (30 min at 37°C). Values are expressed as a percentage of the mean ± SD of that obtained from eNOS exposed to vehicle only (sodium phosphate buffer, pH 7.4, 100%). *, P < 0.05 vs. SPERNO alone.
Fig. 7.
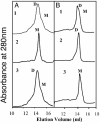
Gel filtration analyses of purified human eNOS from E. coli. (A and B) Human eNOS (200 μg) was subjected to gel filtration analysis in the presence (A1) or absence (A2) of SPERNO (100 μM) for 30 min at 37° and with DTT (5 mM) (A3), thioredoxin (5 μM), and thioredoxin reductase (6.5 μM) and 0.2 mM NADPH (B1), thioredoxin (15 μM), and thioredoxin reductase (19.5 μM) and NADPH (0.6 mM) (B2), or thioredoxin (5 μM) and 0.2 mM NADPH without thioredoxin reductase (B3). NO alone reduces the level of dimeric eNOS, and this effect is dose-dependently diminished in the presence of thioredoxin/thioredoxin reductase and NADPH but not in the absence of thioredoxin reductase. DTT at 5 mM prevents the exogenous NO-induced monomerization (A3). Shown are representative gel filtration analyses from three independent experiments.
Fig. 8.
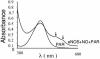
Zn release assay. Purified human eNOS (1 mg) was treated with SPERNO (100 μM) for 30 min at 37°C in a cuvette containing PAR (150 μM). A cuvette containing only eNOS was used to autozero the spectrophotometer. After mixing well, absorbance spectra was recorded between 300–600 nm. Shown is a representative recording from three independent experiments.
Fig. 9.
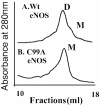
Gel filtration analyses of purified wild-type and C99A mutant eNOS enzyme. Human eNOS (200 μg) or the C99A mutant eNOS protein (200 μg) was subjected to gel filtration analyses. Under identical purification conditions, the eNOS protein is a mixture of both monomers and dimers, but the C99A mutant protein is exclusively monomeric after a freeze–thaw cycle. Shown are representative gel filtration analyses from three independent experiments.
Similar articles
- A new look at the role of nitric oxide in preeclampsia: Protein S-nitrosylation.
Rezeck Nunes P, Cezar Pinheiro L, Zanetoni Martins L, Alan Dias-Junior C, Carolina Taveiros Palei A, Cristina Sandrim V. Rezeck Nunes P, et al. Pregnancy Hypertens. 2022 Aug;29:14-20. doi: 10.1016/j.preghy.2022.05.008. Epub 2022 May 27. Pregnancy Hypertens. 2022. PMID: 35660510 Free PMC article. Review. - Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells.
Erwin PA, Lin AJ, Golan DE, Michel T. Erwin PA, et al. J Biol Chem. 2005 May 20;280(20):19888-94. doi: 10.1074/jbc.M413058200. Epub 2005 Mar 17. J Biol Chem. 2005. PMID: 15774480 - Nitric oxide donors regulate nitric oxide synthase in bovine pulmonary artery endothelium.
Chen JX, Berry LC, Tanner M, Chang M, Myers RP, Meyrick B. Chen JX, et al. J Cell Physiol. 2001 Jan;186(1):116-23. doi: 10.1002/1097-4652(200101)186:1<116::AID-JCP1005>3.0.CO;2-X. J Cell Physiol. 2001. PMID: 11147806 - Nitric oxide activates p21ras and leads to the inhibition of endothelial NO synthase by protein nitration.
Brennan LA, Wedgwood S, Bekker JM, Black SM. Brennan LA, et al. DNA Cell Biol. 2003 May;22(5):317-28. doi: 10.1089/104454903322216662. DNA Cell Biol. 2003. PMID: 12941159 - Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism.
Tenopoulou M, Doulias PT. Tenopoulou M, et al. F1000Res. 2020 Oct 1;9:F1000 Faculty Rev-1190. doi: 10.12688/f1000research.19998.1. eCollection 2020. F1000Res. 2020. PMID: 33042519 Free PMC article. Review.
Cited by
- S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system.
Maron BA, Tang SS, Loscalzo J. Maron BA, et al. Antioxid Redox Signal. 2013 Jan 20;18(3):270-87. doi: 10.1089/ars.2012.4744. Epub 2012 Sep 5. Antioxid Redox Signal. 2013. PMID: 22770551 Free PMC article. Review. - Thioredoxins, glutaredoxins, and glutathionylation: new crosstalks to explore.
Michelet L, Zaffagnini M, Massot V, Keryer E, Vanacker H, Miginiac-Maslow M, Issakidis-Bourguet E, Lemaire SD. Michelet L, et al. Photosynth Res. 2006 Sep;89(2-3):225-45. doi: 10.1007/s11120-006-9096-2. Epub 2006 Nov 7. Photosynth Res. 2006. PMID: 17089213 Review. - A new look at the role of nitric oxide in preeclampsia: Protein S-nitrosylation.
Rezeck Nunes P, Cezar Pinheiro L, Zanetoni Martins L, Alan Dias-Junior C, Carolina Taveiros Palei A, Cristina Sandrim V. Rezeck Nunes P, et al. Pregnancy Hypertens. 2022 Aug;29:14-20. doi: 10.1016/j.preghy.2022.05.008. Epub 2022 May 27. Pregnancy Hypertens. 2022. PMID: 35660510 Free PMC article. Review. - Nitrooleate mediates nitric oxide synthase activation in endothelial cells.
Shin E, Yeo E, Lim J, Chang YH, Park H, Shim E, Chung H, Hwang HJ, Chun J, Hwang J. Shin E, et al. Lipids. 2014 May;49(5):457-66. doi: 10.1007/s11745-014-3893-8. Epub 2014 Mar 25. Lipids. 2014. PMID: 24664541 - eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity.
Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. Rafikov R, et al. J Endocrinol. 2011 Sep;210(3):271-84. doi: 10.1530/JOE-11-0083. Epub 2011 Jun 3. J Endocrinol. 2011. PMID: 21642378 Free PMC article. Review.
References
- Xie, Q. W., Cho, H. J., Calaycay, J., Mumford, R. A., Swiderek, K. M., Lee, T. D., Ding, A., Troso, T. & Nathan, C. (1992) Science 256, 225-228. - PubMed
- Bredt, D. S., Hwang, P. M., Glatt, C. E., Lowenstein, C., Reed, R. R. & Snyder, S. H. (1991) Nature 351, 714-718. - PubMed
- Ghosh, D. K., Abu-Soud, H. M. & Stuehr, D. J. (1996) Biochemistry 35, 1444-1449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD39110/HD/NICHD NIH HHS/United States
- R01 HL070061/HL/NHLBI NIH HHS/United States
- HL67841/HL/NHLBI NIH HHS/United States
- HL72123/HL/NHLBI NIH HHS/United States
- R01 HD039110/HD/NICHD NIH HHS/United States
- R01 HL072123/HL/NHLBI NIH HHS/United States
- R01 HL067841/HL/NHLBI NIH HHS/United States
- HL60190/HL/NHLBI NIH HHS/United States
- R01 HL060190/HL/NHLBI NIH HHS/United States
- HL70061/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources