A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice - PubMed (original) (raw)
A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice
Christine E Paul et al. J Neurosci. 2004.
Abstract
The p75 neurotrophin receptor (p75NTR) regulates neuronal survival, apoptosis, and growth. Recent studies have reported that disruption of Exon IV produces a null mouse lacking all p75NTR gene products (p75NTRExonIV-/-), whereas mice lacking p75NTR Exon III (p75NTRExonIII-/-) maintain expression of an alternatively spliced form of p75NTR (s-p75NTR). Here, we report that p75NTRExonIV-/- mice express a p75NTR gene product that encodes a truncated protein containing the extracellular stalk region together with the entire transmembrane and intracellular domains. The gene product is initiated from a cryptic Kozak consensus/initiator ATG sequence within a region of Exon IV located 3' to the pGK-Neo insertion site. Overexpression of this fragment in heterologous cells results in activation of Jun kinase and induces Pro-caspase-3 cleavage, indicating that it activates p75NTR signaling cascades. These results indicate that aspects of the p75NTRExonIV-/- phenotype may reflect a gain-of-function mutation rather than loss of p75NTR function.
Figures
Figure 1.
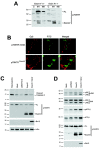
A p75NTR fragment is expressed in p75NTRExonIV-/- mice. A, P1 brain lysates from p75NTRExonIII and p75NTRExonIV wild-type (+/+), heterozygous (+/-), and null (-/-) littermates were separated on a 15% SDS-PAGE gel, followed by immunoblotting with αP1 (top panel) or αp75NTR (middle panel), distinct polyclonal antibodies directed against the p75NTR-ICD (see Materials and Methods). Arrowheads indicate full-length p75NTR and a ∼26 kDa band present in lysates from mice bearing the p75NTRExonIV mutant allele. Blots were reprobed for βIII-tubulin to verify equal loading. B, P1 cerebellar and heart lysates from p75NTRExonIV wild-type (+/+), heterozygous (+/-), and null (-/-) littermates were prepared and subjected to SDS-PAGE and immunoblotting as described above. C, Schematic diagram showing the genomic structure of the mouse p75NTR locus and corresponding protein domains. D, RNA was isolated from P1 brain lysates of p75NTRExonIV wild-type (+/+), heterozygous (+/-), and null (-/-) littermates. cDNA was prepared in the presence (+RT) and absence (-RT) of reverse transcriptase, followed by RT-PCR using primers for β-actin and for p75NTR-ICD. Arrows in C indicate location of primers used for this analysis. Experiments in A, B, and D were repeated three times with identical results.
Figure 2.
Transcription of p75NTR fragment in p75NTRExonIV-/- mice is the result of pGK-Neo cassette insertion into the p75NTRExonIV targeting vector. A, mRNA from p75NTRExonIV wild-type (+/+), heterozygous (+/-), and null (-/-) littermates was subjected to RT-PCR using primers that target regions of p75NTR upstream or downstream of the pGK-Neo cassette insertion in the p75NTRExonIV targeting vector, as indicated in the schematic diagram. B, Alignment of mouse p75NTR cDNA sequence with p75NTR transcript cloned from p75NTRExonIV-/- mice. The putative start codon is underlined, and the putative Kozak sequence is boxed. Vertical lines indicate Exon IV-V and Exon V-VI junctions, and boxes underneath the sequence denote protein domains. C, Schematic diagram showing the protein domains of mouse p75NTR and p75NTRExonIV proteins. D, P1 brain lysates from p75NTRExonIV wild-type (+/+), heterozygous (+/-), and null (-/-) littermates and cellular lysates from 293T cells transiently transfected with plasmids encoding p75NTRExonIV (ExonIV) or the parental vector (Mock) were immunoblotted using αP1. Arrowheads indicate full-length p75NTR (top) and p75NTRExonIV (bottom) products. Experiments in A and D were repeated three times with identical results.
Figure 3.
p75NTRExonIV is membrane-associated and induces p75NTR signaling events. A, P1 brains from p75NTRExonIV heterozygote (Exon IV +/-) and null (Exon IV -/-) littermates were subjected to subcellular fractionation and analyzed by immunoblot. C, Cytosol; M1, heavy membrane; M2, light membrane fraction. B, COS-7 cells were transfected with plasmids encoding p75NTRExonIV or the p75NTR intracellular domain tagged with a myristoylation sequence (Roux et al., 2001) and immunostained with αP1 and α-nucleolin. Left-hand panels show p75NTR staining, middle panels show nucleolin staining, and right-hand panels show merged images. C, PC12 cells were transfected with control plasmid or plasmid expressing p75NTRExonIV in the presence or absence of plasmid expressing Bcl-2. Staurosporine treatment was used as a positive control for caspase cleavage. Cellular lysates were analyzed by immunoblot to detect p75NTR, Bcl-2, and active caspase-3, as indicated. IκBα levels were analyzed to confirm equal loading between lanes. D, PC12 cells were left untransfected or were transfected with 2 μg of a control plasmid (pcDNA3), or expression plasmids encoding p75NTRExonIV alone or p75NTRExonIV and Bcl-2 and levels of phospho-Thr183/Tyr185-JNK and total JNK, phospho-Thr69/71-ATF-2, and total ATF-2 were assessed by immunoblotting. Experiments in _A_-C were repeated three times with identical results.
Similar articles
- Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.
Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Culmsee C, et al. Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482 - Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability.
Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Kanning KC, et al. J Neurosci. 2003 Jul 2;23(13):5425-36. doi: 10.1523/JNEUROSCI.23-13-05425.2003. J Neurosci. 2003. PMID: 12843241 Free PMC article. - Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad.
Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA. Bhakar AL, et al. J Neurosci. 2003 Dec 10;23(36):11373-81. doi: 10.1523/JNEUROSCI.23-36-11373.2003. J Neurosci. 2003. PMID: 14673001 Free PMC article. - p75NTR: A study in contrasts.
Barker PA. Barker PA. Cell Death Differ. 1998 May;5(5):346-56. doi: 10.1038/sj.cdd.4400375. Cell Death Differ. 1998. PMID: 10200483 Review. - The Molecular Pathway of p75 Neurotrophin Receptor (p75NTR) in Parkinson's Disease: The Way of New Inroads.
Ali NH, Al-Kuraishy HM, Al-Gareeb AI, Alnaaim SA, Saad HM, Batiha GE. Ali NH, et al. Mol Neurobiol. 2024 May;61(5):2469-2480. doi: 10.1007/s12035-023-03727-8. Epub 2023 Oct 28. Mol Neurobiol. 2024. PMID: 37897634 Review.
Cited by
- Sexual Dimorphism in Balance and Coordination in p75NTRexonIII Knock-Out Mice.
Abbasian M, Langlois A, Gibon J. Abbasian M, et al. Front Behav Neurosci. 2022 Feb 24;16:842552. doi: 10.3389/fnbeh.2022.842552. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35283743 Free PMC article. - Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons.
Zhang YH, Vasko MR, Nicol GD. Zhang YH, et al. J Physiol. 2006 Aug 15;575(Pt 1):101-13. doi: 10.1113/jphysiol.2006.111575. Epub 2006 Jun 1. J Physiol. 2006. PMID: 16740613 Free PMC article. - Brain-derived neurotrophic factor: a newly described mediator of angiogenesis.
Kermani P, Hempstead B. Kermani P, et al. Trends Cardiovasc Med. 2007 May;17(4):140-3. doi: 10.1016/j.tcm.2007.03.002. Trends Cardiovasc Med. 2007. PMID: 17482097 Free PMC article. Review. - Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles.
Vilar M, Mira H. Vilar M, et al. Front Neurosci. 2016 Feb 9;10:26. doi: 10.3389/fnins.2016.00026. eCollection 2016. Front Neurosci. 2016. PMID: 26903794 Free PMC article. Review. - Saikosaponin-d impedes hippocampal neurogenesis and causes cognitive deficits by inhibiting the survival of neural stem/progenitor cells via neurotrophin receptor signaling in mice.
Qin T, Yuan Z, Yu J, Fu X, Deng X, Fu Q, Ma Z, Ma S. Qin T, et al. Clin Transl Med. 2020 Dec;10(8):e243. doi: 10.1002/ctm2.243. Clin Transl Med. 2020. PMID: 33377633 Free PMC article.
References
- Barrett GL (2000) The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol 61: 205-229. - PubMed
- Davies AM, Lee KF, Jaenisch R (1993) p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron 11: 565-574. - PubMed
- Eilers A, Whitfield J, Shah B, Spadoni C, Desmond H, Ham J (2001) Direct inhibition of c-Jun N-terminal kinase in sympathetic neurones prevents c-jun promoter activation and NGF withdrawal-induced death. J Neurochem 76: 1439-1454. - PubMed
- Frankowski H, Castro-Obregon S, del Rio G, Rao RV, Bredesen DE (2002) PLAIDD, a type II death domain protein that interacts with p75 neurotrophin receptor. Neuromol Med 1: 153-170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous