RUNX3 expression in primary and metastatic pancreatic cancer - PubMed (original) (raw)
RUNX3 expression in primary and metastatic pancreatic cancer
J Li et al. J Clin Pathol. 2004 Mar.
Abstract
Aim: Runx transcription factors are important regulators of lineage specific gene expression, cell proliferation, and differentiation. Runx3 expression is lost in a high proportion of gastric cancers, suggesting a tumour suppressive role in this malignancy. This study investigates the expression and localisation of Runx3 in pancreatic tissues.
Methods: Quantitative polymerase chain reaction was used to measure Runx3 mRNA. Immunohistochemistry was carried out to localise Runx3 in normal pancreatic tissues, and in primary and metastatic pancreatic ductal adenocarcinoma (PDAC). Basal and transforming growth factor beta1 (TGFbeta1) induced Runx3 expression was analysed in cultured pancreatic cancer cell lines.
Results: Runx3 expression was low to absent in normal pancreatic tissues, but increased in a third of cancer tissues. Runx3 was present only in islets in normal pancreas, whereas in pancreatic cancers, Runx3 was detected in the cancer cells of seven of 24 samples analysed. In addition, it was expressed by lymphocytes in six of the 16 cases with lymphocyte infiltration. In pancreatic cancer cell lines, Runx3 mRNA was present in Colo-357 and T3M4 cells, but was low to absent in the other cell lines tested. TGFbeta1 repressed Runx3 mRNA expressed in Colo-357 cells, and had no effect on Runx3 expression in the other pancreatic cancer cell lines.
Conclusion: Runx3 expression is restricted to islets in the normal pancreas. In contrast, a considerable proportion of pancreatic tumours express Runx3, and its expression is localised in the tumour cells and in the infiltrating lymphocytes. Thus, Runx3 might play a role in the pathogenesis of PDAC.
Figures
Figure 1
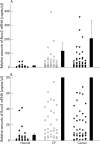
Runx3 mRNA expression in pancreatic tissues: Runx3 mRNA values in normal pancreatic tissues, chronic pancreatitis (CP), and pancreatic ductal adenocarcinoma (cancer) tissues by real time quantitative polymerase chain reaction, as described in the Methods section. Values were normalised to housekeeping genes (cyclophilin B and hypoxanthine guanine phosphoribosyltransferase), and are presented as mean ± SEM. Note the different scales in A and B, which better illustrates the differences in expression.
Figure 2
Runx3 localisation in pancreatic tissues: immunohistochemistry using a Runx3 specific antibody was carried out in (A) the normal pancreas and (B–F) pancreatic cancers. (B) A moderately stained pancreatic cancer with infiltrating inflammatory cells; (C, D) Runx3 positive cancers; (E, F) Runx3 negative pancreatic cancers.
Figure 3
Runx3 localisation in primary and metastatic pancreatic cancer tissues: immunohistochemistry of Runx3 in (A and B) primary pancreatic cancers, (C) liver metastasis, and (D) lymph node metastasis. (A) Runx3 negative lymphocytes; (B) Runx3 immunoreactivity in some of the lymphocytes. (D) Note also some Runx3 positive lymphocytes surrounding the lymph node metastasis.
Figure 4
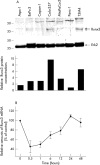
Runx3 expression and regulation in pancreatic cancer cell lines: (A) protein lysates (20 µg) of the indicated cell lines were subjected to Western blotting using a Runx3 antibody, as described in the Methods section (upper panel). Equal loading was determined by stripping the membrane and blotting with an antibody to Erk2 (middle panel). Relative amounts of Runx3 protein were determined by OD (Runx3)/OD (Erk2) × 100 for each sample (lower panel). (B) Colo-357 cells were incubated in the absence (0) or presence of 200pM transforming growth factor β1 for the indicated time. RNA was extracted and real time quantitative polymerase chain reaction for Runx3 was performed. The respective control mRNA expression values were set to 100%. Results are presented as mean ± SEM of three independent experiments. OD, optical density.
Similar articles
- Yes-associated protein (YAP65) in relation to Smad7 expression in human pancreatic ductal adenocarcinoma.
Guo J, Kleeff J, Zhao Y, Li J, Giese T, Esposito I, Büchler MW, Korc M, Friess H. Guo J, et al. Int J Mol Med. 2006 May;17(5):761-7. Int J Mol Med. 2006. PMID: 16596258 - Frequent loss of RUNX3 gene expression in human bile duct and pancreatic cancer cell lines.
Wada M, Yazumi S, Takaishi S, Hasegawa K, Sawada M, Tanaka H, Ida H, Sakakura C, Ito K, Ito Y, Chiba T. Wada M, et al. Oncogene. 2004 Mar 25;23(13):2401-7. doi: 10.1038/sj.onc.1207395. Oncogene. 2004. PMID: 14743205 - Tenascin C and annexin II expression in the process of pancreatic carcinogenesis.
Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P. Esposito I, et al. J Pathol. 2006 Apr;208(5):673-85. doi: 10.1002/path.1935. J Pathol. 2006. PMID: 16450333 - Runx3 and Cell Fate Decisions in Pancreas Cancer.
Whittle MC, Hingorani SR. Whittle MC, et al. Adv Exp Med Biol. 2017;962:333-352. doi: 10.1007/978-981-10-3233-2_21. Adv Exp Med Biol. 2017. PMID: 28299667 Review. - Estrogens and pancreatic cancer: some recent aspects.
Andrén-Sandberg A. Andrén-Sandberg A. Scand J Gastroenterol. 1986 Mar;21(2):129-33. doi: 10.3109/00365528609034636. Scand J Gastroenterol. 1986. PMID: 3520793 Review. No abstract available.
Cited by
- Molecular mechanisms of pancreatic carcinogenesis.
Furukawa T, Sunamura M, Horii A. Furukawa T, et al. Cancer Sci. 2006 Jan;97(1):1-7. doi: 10.1111/j.1349-7006.2005.00134.x. Cancer Sci. 2006. PMID: 16367914 Free PMC article. Review. - Loss of runt-related transcription factor 3 expression leads hepatocellular carcinoma cells to escape apoptosis.
Nakanishi Y, Shiraha H, Nishina S, Tanaka S, Matsubara M, Horiguchi S, Iwamuro M, Takaoka N, Uemura M, Kuwaki K, Hagihara H, Toshimori J, Ohnishi H, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yagi T, Yamamoto K. Nakanishi Y, et al. BMC Cancer. 2011 Jan 4;11:3. doi: 10.1186/1471-2407-11-3. BMC Cancer. 2011. PMID: 21205319 Free PMC article. - A novel HMM-based method for detecting enriched transcription factor binding sites reveals RUNX3 as a potential target in pancreatic cancer biology.
Levkovitz L, Yosef N, Gershengorn MC, Ruppin E, Sharan R, Oron Y. Levkovitz L, et al. PLoS One. 2010 Dec 22;5(12):e14423. doi: 10.1371/journal.pone.0014423. PLoS One. 2010. PMID: 21203558 Free PMC article. - Runt-related transcription factors in human carcinogenesis: a friend or foe?
Roy A, Chauhan S, Bhattacharya S, Jakhmola V, Tyagi K, Sachdeva A, Wasai A, Mandal S. Roy A, et al. J Cancer Res Clin Oncol. 2023 Sep;149(11):9409-9423. doi: 10.1007/s00432-023-04769-0. Epub 2023 Apr 21. J Cancer Res Clin Oncol. 2023. PMID: 37081242 Review. - Lineage-specific RUNX3 hypomethylation marks the preneoplastic immune component of gastric cancer.
Kurklu B, Whitehead RH, Ong EK, Minamoto T, Fox JG, Mann JR, Judd LM, Giraud AS, Menheniott TR. Kurklu B, et al. Oncogene. 2015 May 28;34(22):2856-66. doi: 10.1038/onc.2014.233. Epub 2014 Aug 4. Oncogene. 2015. PMID: 25088199 Free PMC article.
References
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23–47. - PubMed
- Kern SE, Hruban RH, Hidalgo M, et al. An introduction to pancreatic adenocarcinoma genetics, pathology and therapy. Cancer Biol Ther 2002;1:607–13. - PubMed
- Ozawa F, Friess H, Tempia-Caliera A, et al. Growth factors and their receptors in pancreatic cancer. Teratog Carcinog Mutagen 2001;21:27–44. - PubMed
- Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol 2000;11:343–6. - PubMed
- Tracey WD, Speck NA. Potential roles for RUNX1 and its orthologs in determining hematopoietic cell fate. Semin Cell Dev Biol 2000;11:337–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous