Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A - PubMed (original) (raw)
Comparative Study
Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A
Takashi Irie et al. J Virol. 2004 Mar.
Erratum in
- J Virol. 2004 May;78(10):5532
Abstract
Viral matrix proteins of several enveloped RNA viruses play important roles in virus assembly and budding and are by themselves able to bud from the cell surface in the form of lipid-enveloped, virus-like particles (VLPs). Three motifs (PT/SAP, PPxY, and YxxL) have been identified as late budding domains (L-domains) responsible for efficient budding. L-domains can functionally interact with cellular proteins involved in vacuolar sorting (VPS4A and TSG101) and endocytic pathways (Nedd4), suggesting involvement of these pathways in virus budding. Ebola virus VP40 has overlapping PTAP and PPEY motifs, which can functionally interact with TSG101 and Nedd4, respectively. As for vesicular stomatitis virus (VSV), a PPPY motif within M protein can interact with Nedd4. In addition, M protein has a PSAP sequence downstream of the PPPY motif, but the function of PSAP in budding is not clear. In this study, we compared L-domain functions between Ebola virus and VSV by constructing a chimeric M protein (M40), in which the PPPY motif of VSV M is replaced by the L domains of VP40. The budding efficiency of M40 was 10-fold higher than that of wild-type (wt) M protein. Overexpression of a dominant negative mutant of VPS4A or depletion of cellular TSG101 reduced the budding of only M40-containing VLPs but not that of wt M VLPs or live VSV. These findings suggest that the PSAP motif of M protein is not critical for budding and that there are fundamental differences between PTAP-containing viruses (Ebola virus and human immunodeficiency virus type 1) and PPPY-containing viruses (VSV and rabies virus) regarding their dependence on specific host factors for efficient budding.
Figures
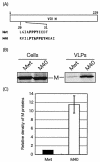
FIG. 1.
Budding assay of VSV M proteins. (A) Schematic representation of expression plasmids encoding Mwt and chimeric M40. The amino acid sequences of the L-domains are indicated by their single-letter code. (B) Radiolabeled cell lysates and VLPs were immunoprecipitated with anti-M MAb, and immunoprecipitates were analyzed by SDS-PAGE. (C) M proteins in VLPs were quantitated by densitometry with the ImageJ 1.24 software, and the bars represent an average of six independent experiments. The level of Mwt was normalized to 1.0, and the relative value of M40 is indicated.
FIG. 2.
Effect of DN VPS4A on budding of VP40-WT and VP40-dPTA VLPs. Human 293T cells were cotransfected with a combination of VP40-WT or VP40-dPTA and the indicated VPS4A plasmid (wt or DN EQ mutant). Cell lysates (A) and VLPs (B) were immunoprecipitated with the appropriate antibodies to detect VP40 or VPS4A as indicated. VP40 in VLPs was quantitated by PhosphorImager analysis.
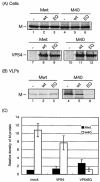
FIG. 3.
Effect of DN VPS4A on budding of VSV Mwt and M40 VLPs. BHK-21 cells were infected with VvT7 and then cotransfected with the indicated plasmids. Cell lysates (A) and VLPs (B) were immunoprecipitated with the appropriate antibodies to detect VSV M or VPS4A. (C) Mwt and M40 present in VLPs were quantitated, and the bars represent an average of three independent experiments. The level of Mwt in VLP samples from cells not receiving VPS4A (−) was normalized to 1.0.
FIG. 4.
Effect of DN VPS4A on VSV budding. (A and B) COS-1 cells were transfected with vector alone (−), VPS4-WT (wt), or VPS4-EQ (EQ). At 24 h p.t., the cells were infected with VSV at an MOI of 0.1 PFU/ml, and culture medium was harvested at 0, 4, 6, 8, 10, and 12 h p.i. Virions (A) and cell lysates (B) were harvested at 12 h p.i. M protein was immunoprecipitated from virions (A), and both M and VPS4A proteins (B) were immunoprecipitated from cell extracts. (C) The virus titer was determined by plaque assay, and titers were plotted against time p.i.
FIG. 5.
Growth kinetics of recombinant RV expressing VPS4A proteins. (A) Schematic representation of the genome of WT SPBN and recombinant genomes containing VPS4-WT, VPS4-EQ, and VPS4-KQ. All inserts and 5′ and 3′ promoter regions were sequenced. (B) BSR-T7 cells were infected with the indicated viruses at an MOI of 0.01 PFU/ml, and culture medium was harvested at 8, 24, 48, and 72 h p.i. Virus titer at each time point was determined in duplicate on BSR cells and is plotted against time p.i.
FIG. 6.
Effect of TSG101 depletion on budding of Mwt and M40 VLPs. (A) Human 293T cells were transfected with TSG101-specific or nonspecific (NS) siRNAs together with a TSG101-encoding plasmid (pCAGGS.MCS vector). TSG101 was detected by immunoprecipitation with anti-TSG101 antibody. (B) Immunoprecipitation of Mwt and M40 proteins from cell extracts (lanes 1 to 6) and VLPs (lanes 7 to 12) in the absence of siRNA, in the presence of NS siRNA, or in the presence of TSG101 siRNA as indicated. (C) Quantitation of Mwt and M40 in VLPs from three independent experiments. The level of Mwt protein in VLP samples from NS siRNA-transfected cells was normalized to 1.0.
FIG. 7.
Effect of TSG101 depletion on VSV budding. Human 293T cells were transfected with TSG101-specific or NS siRNAs as indicated and then infected with VSV at an MOI of 0.1 PFU/ml. M protein present in cell lysates and virions was detected by immunoprecipitation. Virus production (10 h p.i.) from cells receiving TSG101-specific or NS siRNA was determined by a duplicate plaque assay on BHK-21 cells.
Similar articles
- Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4.
Licata JM, Simpson-Holley M, Wright NT, Han Z, Paragas J, Harty RN. Licata JM, et al. J Virol. 2003 Feb;77(3):1812-9. doi: 10.1128/jvi.77.3.1812-1819.2003. J Virol. 2003. PMID: 12525615 Free PMC article. - The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release.
Gottwein E, Bodem J, Müller B, Schmechel A, Zentgraf H, Kräusslich HG. Gottwein E, et al. J Virol. 2003 Sep;77(17):9474-85. doi: 10.1128/jvi.77.17.9474-9485.2003. J Virol. 2003. PMID: 12915562 Free PMC article. - ALIX Rescues Budding of a Double PTAP/PPEY L-Domain Deletion Mutant of Ebola VP40: A Role for ALIX in Ebola Virus Egress.
Han Z, Madara JJ, Liu Y, Liu W, Ruthel G, Freedman BD, Harty RN. Han Z, et al. J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S138-45. doi: 10.1093/infdis/jiu838. Epub 2015 Mar 18. J Infect Dis. 2015. PMID: 25786915 Free PMC article. - [Envelope virus assembly and budding].
Irie T. Irie T. Uirusu. 2010 Jun;60(1):105-13. doi: 10.2222/jsv.60.105. Uirusu. 2010. PMID: 20848870 Review. Japanese. - Rhabdovirus assembly and budding.
Jayakar HR, Jeetendra E, Whitt MA. Jayakar HR, et al. Virus Res. 2004 Dec;106(2):117-32. doi: 10.1016/j.virusres.2004.08.009. Virus Res. 2004. PMID: 15567492 Review.
Cited by
- Amino acid residues in the cytoplasmic domain of the Mason-Pfizer monkey virus glycoprotein critical for its incorporation into virions.
Song C, Micoli K, Bauerova H, Pichova I, Hunter E. Song C, et al. J Virol. 2005 Sep;79(18):11559-68. doi: 10.1128/JVI.79.18.11559-11568.2005. J Virol. 2005. PMID: 16140733 Free PMC article. - Status of antiviral therapeutics against rabies virus and related emerging lyssaviruses.
Du Pont V, Plemper RK, Schnell MJ. Du Pont V, et al. Curr Opin Virol. 2019 Apr;35:1-13. doi: 10.1016/j.coviro.2018.12.009. Epub 2019 Feb 10. Curr Opin Virol. 2019. PMID: 30753961 Free PMC article. Review. - Paramyxovirus assembly and budding: building particles that transmit infections.
Harrison MS, Sakaguchi T, Schmitt AP. Harrison MS, et al. Int J Biochem Cell Biol. 2010 Sep;42(9):1416-29. doi: 10.1016/j.biocel.2010.04.005. Epub 2010 Apr 14. Int J Biochem Cell Biol. 2010. PMID: 20398786 Free PMC article. Review. - Budding of Enveloped Viruses: Interferon-Induced ISG15-Antivirus Mechanisms Targeting the Release Process.
Seo EJ, Leis J. Seo EJ, et al. Adv Virol. 2012;2012:532723. doi: 10.1155/2012/532723. Epub 2012 May 17. Adv Virol. 2012. PMID: 22666250 Free PMC article. - L-domain flanking sequences are important for host interactions and efficient budding of vesicular stomatitis virus recombinants.
Irie T, Harty RN. Irie T, et al. J Virol. 2005 Oct;79(20):12617-22. doi: 10.1128/JVI.79.20.12617-12622.2005. J Virol. 2005. PMID: 16188963 Free PMC article.
References
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. - PubMed
- Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland, and S. D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:283-289. - PubMed
- Babst, M., G. Odorizzi, E. J. Estepa, and S. D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248-258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007324/AI/NIAID NIH HHS/United States
- AI49153/AI/NIAID NIH HHS/United States
- R21 AI049153/AI/NIAID NIH HHS/United States
- T32-AI007324/AI/NIAID NIH HHS/United States
- R01 AI049153/AI/NIAID NIH HHS/United States
- AI46499/AI/NIAID NIH HHS/United States
- R01 AI046499/AI/NIAID NIH HHS/United States
- AI53393/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous