Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors - PubMed (original) (raw)
Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors
Clare E Thomas et al. J Virol. 2004 Mar.
Abstract
Transduction of the liver with single-stranded adeno-associated virus serotype 2 (AAV2) vectors is inefficient; less than 10% of hepatocytes are permissive for stable transduction, and transgene expression is characterized by a lag phase of up to 6 weeks. AAV2-based vector genomes packaged inside AAV6 or AAV8 capsids can transduce the liver with higher efficiency, but the molecular mechanisms underlying this phenomenon have not been determined. We now show that the primary barrier to transduction of the liver with vectors based on AAV2 capsids is uncoating of vector genomes in the nucleus. The majority of AAV2 genomes persist as encapsidated single-stranded molecules within the nucleus for as long as 6 weeks after vector administration. Double-stranded vector genomes packaged inside AAV2 capsids are at least 50-fold more active than single-stranded counterparts, but these vectors also exhibit a lag phase before maximal gene expression. Vector genomes packaged inside AAV6 or AAV8 capsids do not persist as encapsidated molecules and are more biologically active than vector genomes packaged inside AAV2 capsids. Our data suggest that the rate of uncoating of vector genomes determines the ability of complementary plus and minus single-stranded genomes to anneal together and convert to stable, biologically active double-stranded molecular forms.
Figures
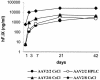
FIG. 1.
Plasma hF.IX levels after intraportal administration of 1011 VG of AAV-hF.IX16 pseudotypes in C57BL/6 mice. The AAV pseudotypes contained an hF.IX expression cassette flanked by AAV2 ITRs packaged inside an AAV2 capsid (AAV2/2), an AAV6 capsid (AAV2/6), or an AAV8 capsid (AAV2/8). Vectors were purified by cesium chloride gradient centrifugation (CsCl) or heparin sulfate column chromatography (HPLC).
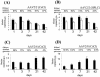
FIG. 2.
X-Gal histochemistry of liver sections from immune-deficient C57BL/6 Rag-1 mice 6 weeks after intraportal delivery of _lacZ_-expressing vector pseudotypes. (a) Mice were injected with 3 × 1011 VG of AAV2/2-EF1α-nlslacZ. A section from a representative mouse is shown. (b and c) Sections from mice injected with 3 × 1011 VG (b) or 1.8 × 1012 VG (c) of AAV2/8-EF1α-nlslacZ. Scale bars in panels a to c represent 200 mm. (d) The boxed area in panel c.
FIG. 3.
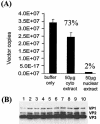
Real-time PCR quantitation of the proportions of DNase I-resistant AAV genomes localized within the nucleus over time. Numbers of AAV genomes were quantified in total DNA extracted from purified liver nuclei prepared at different time points after intraportal injection of 1011 VG of pseudotyped AAV-hF.IX16 vectors into C57BL/6 mice. Solid black bars indicate the total number of nuclear-localized AAV genomes at each time point (expressed as copy numbers per DGE), and open bars indicate the relative number of DNase I-resistant AAV genomes. The ratio of DNase I-resistant to total nuclear-localized VG at each time point is expressed as a percentage above each graph. n = 4 mice per vector per time point. Error bars show standard errors of the means.
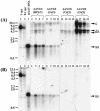
FIG. 4.
Southern blot analysis of rAAV VG extracted from purified liver nuclei without preincubation with DNase I (A), or after incubation with DNase I for 5 h at 37°C (B). Vector forms in DNA samples extracted 3 weeks after injection of pseudotyped AAV-hF.IX16 vectors are shown. Forty micrograms of undigested total DNA (A) or the equivalent volume of DNase I-treated sample (B) was separated on a 1% agarose gel, blotted, and probed with a vector sequence-specific probe. Lanes 1 and 2, 1- and 0.1-VG/DGE standards, respectively (40 μg of DNA extracted from naïve mice was spiked with a 6.4-kb plasmid containing the AAVhF.IX16 sequence and then digested with _Sac_I prior to loading). A total of 107 VG of AAV-hF.IX16 extracted from purified vector stock was denatured by boiling for 5 min in the presence of formamide and loaded in lane 3 as a size marker for ss AAV-hF.IX genomes. Lanes 5 to 15 represent individual mice. Arrowheads indicate the different molecular forms of the AAV-hF.IX16 genome. ds indicates either relaxed circular, supercoiled circular, or linear ds forms. c, concatemers.
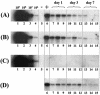
FIG. 5.
Immunoprecipitation of intact AAV2/2 particles from purified liver nuclei. Liver nuclei purified at different time points after intraportal injection of 5 × 1011 VG of AAV2/2-hF.IX16 into C57BL/6 mice were incubated with DNase I for 1 h at 37°C. (A and B) Intact AAV capsids were immunoprecipitated from solubilized, DNase I-treated nuclei using the A20 antibody (A) or heparin-Sepharose beads (B). Immunoprecipitated capsids were boiled for 5 min in alkaline buffer, and the released VG were separated on a 1% alkaline agarose gel, blotted, and probed with a sequence-specific probe. (C) Control immunoprecipitations were performed with the anti-VP1,2,3 antibody, which recognizes dissociated, but not intact, capsid proteins. Positive and negative controls were performed for immunoprecipitations with all antibodies. For the positive control, purified nuclei were spiked with 109 VG of AAV2/2-hF.IX.16 prior to solubilization (lane 6). For the negative control, solubilized nuclei were spiked with 1010 VG of AAV-hF.IX16 DNA extracted from purified AAV2/2-hF.IX16 particles (lane 5). Lanes 1 to 4 show copy number standards (purified AAV2/2-hF.IX particles boiled in alkaline buffer prior to loading). Lanes 7 to 15 represent individual mice. (D) Ten microliters of supernatant from solubilized nuclei (from a total volume of 1 ml for each mouse) removed prior to immunoprecipitation was also boiled in alkaline buffer and loaded on a gel.
FIG. 6.
Real-time PCR quantitation of DNase I-resistant genomes after incubation with nuclear extract or cytoplasmic extract. A total of 1010 particles of purified AAV2/2-hF.IX vector were incubated for 30 min at 37°C with 50 μg of nuclear extract, cytoplasmic extract, or the same volume of buffer alone, before digesting with DNase I for a further hour at 37°C (final volume, 100 μl). (A) DNase I-resistant VG in 5 μl were quantified by real-time PCR. Error bars show the standard error of the mean of four samples per group. (B) Twenty microliters of the remaining sample was analyzed by Western blotting with the anti-VP1,2,3 antibody to detect the integrity of the capsid proteins following incubation with nuclear or cytoplasmic extract. Lanes 1 and 2, AAV particles incubated with buffer only; lanes 3 to 6, AAV particles incubated with cytoplasmic extract; lanes 7 to 10, AAV particles incubated with nuclear extract.
FIG. 7.
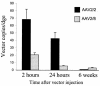
Real-time PCR quantitation of AAV genomes present in genomic DNA extracted from liver tissue. C57BL/6 mice were injected with 1011 VG of AAV2/2.hF.IX-16 or AAV2/8.hF.IX-16, and livers were harvested 2 or 24 h or 6 weeks after vector injection.
FIG. 8.
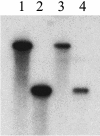
Purity of sc (lane 3) and ss (lane 4) AAV.luc vectors after cesium chloride gradient fractionation. Aliquots of cesium chloride gradient fractions containing sc AAV.luc (refractive index, 1.3736) or ss AAV.luc (refractive index, 1.3702) were electrophoresed on a 1% alkaline agarose gel, Southern blotted, and hybridized with a vector-specific probe. The sc genome migrated with the 4.8-kb marker (lane 1), whereas the ss genome migrated with the 2.4-kb marker (lane 2).
FIG. 9.
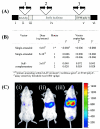
A time course of expression from sc versus ss AAV2/2 vectors expressing luciferase. BALB/c mice (n = 2 per virus) were injected with 5 × 109 VG of sc AAV2/2.luc (▪), 5 × 1010 VG of ss AAV2/2.luc (▴), or 5 × 109 VG of ss AAV2/2.luc (•). (A) Mean luciferase expression for all three virus groups plotted on a log scale. Luciferase expression from the sc vector was up to 50-fold higher than luciferase expression from the same dose of ss vector and remained high over the 1-year period of study. (B to D) Luciferase expression from sc vectors as well as ss vectors gradually increased over the first 5 weeks after vector injection. The expression profiles for two individual animals per virus dose are shown.
FIG. 10.
Real-time PCR quantitation of sc and ss AAV.luc genomes in genomic DNA extracted from the livers of BALB/c mice 1 year after vector injection. (A) sc or ss AAV.luc VG were quantified using three sets of primer-probe combinations. ss VG were undetectable in mice that had been injected with 5 × 109 or 5 × 1010 VG. (B) In contrast, sc VG were reproducibly detected with all three primer-probe sets, even though only the low dose of 5 × 109 VG had been injected. (C) Luciferase expression in three representative mice 1 year after vector injection. (i) Mouse injected with 5 × 109 VG of ss AAV.luv; (ii) mouse injected with 5 × 1010 VG of ss AAV.luc; (iii) mouse injected with 5 × 109 VG of SC AAV.luc. One of the mice in the group injected with 5 × 109 VG of the ss vector died before the 1-year time point.
FIG. 11.
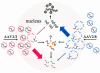
Theoretical model for rAAV transduction of the liver. AAV2 VG packaged within an AAV2 shell (AAV2/2) traffic slowly to the nucleus. Capsid disassembly is mediated by a nuclear factor(s). Upon uncoating, ss VG either become stabilized by annealing rapidly with complementary AAV genomes or they are degraded and lost. The rate of uncoating determines the proportion of VG that become stabilized by annealing or lost by degradation. The AAV2/2 pseudotype uncoats slowly. Annealing is therefore inefficient, and most of the VG are removed by degradation. A nuclear protein (such as FKBP52) might bind to the ss AAV genomes and physically obstruct annealing or target the genome for degradation. The AAV2/8 pseudotype, containing the AAV2 genome packaged within an AAV2/8 capsid, is more efficient than the AAV2/2 pseudotype because it uncoats rapidly. Annealing of complementary genomes occurs with higher efficiency, and a reduced proportion of VG are lost by degradation. Thus, AAV2/8 vectors mediate more efficient liver transduction than AAV2/2 vectors.
Similar articles
- Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses.
Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, Li B, Herzog RW, Srivastava A. Jayandharan GR, et al. Hum Gene Ther. 2010 Mar;21(3):271-83. doi: 10.1089/hum.2009.100. Hum Gene Ther. 2010. PMID: 19788390 Free PMC article. - Transduction of pancreatic islets with pseudotyped adeno-associated virus: effect of viral capsid and genome conversion.
Zhang N, Clément N, Chen D, Fu S, Zhang H, Rebollo P, Linden RM, Bromberg JS. Zhang N, et al. Transplantation. 2005 Sep 15;80(5):683-90. doi: 10.1097/01.tp.0000173381.97556.0d. Transplantation. 2005. PMID: 16177645 - AAV8 capsid variable regions at the two-fold symmetry axis contribute to high liver transduction by mediating nuclear entry and capsid uncoating.
Tenney RM, Bell CL, Wilson JM. Tenney RM, et al. Virology. 2014 Apr;454-455:227-36. doi: 10.1016/j.virol.2014.02.017. Epub 2014 Mar 13. Virology. 2014. PMID: 24725949 - Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes.
Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Halbert CL, et al. J Virol. 2000 Feb;74(3):1524-32. doi: 10.1128/jvi.74.3.1524-1532.2000. J Virol. 2000. PMID: 10627564 Free PMC article. - Adeno-Associated Virus and Hematopoietic Stem Cells: The Potential of Adeno-Associated Virus Hematopoietic Stem Cells in Genetic Medicines.
Chatterjee S, Sivanandam V, Wong KK , Jr. Chatterjee S, et al. Hum Gene Ther. 2020 May;31(9-10):542-552. doi: 10.1089/hum.2020.049. Hum Gene Ther. 2020. PMID: 32253938 Free PMC article. Review.
Cited by
- Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against post-myocardial infarction left ventricular remodeling.
Konkalmatt PR, Beyers RJ, O'Connor DM, Xu Y, Seaman ME, French BA. Konkalmatt PR, et al. Circ Cardiovasc Imaging. 2013 May 1;6(3):478-86. doi: 10.1161/CIRCIMAGING.112.000320. Epub 2013 Mar 27. Circ Cardiovasc Imaging. 2013. PMID: 23536266 Free PMC article. - Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer.
Pipe S, Leebeek FWG, Ferreira V, Sawyer EK, Pasi J. Pipe S, et al. Mol Ther Methods Clin Dev. 2019 Sep 10;15:170-178. doi: 10.1016/j.omtm.2019.08.015. eCollection 2019 Dec 13. Mol Ther Methods Clin Dev. 2019. PMID: 31660419 Free PMC article. Review. - Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype.
Grimm D, Pandey K, Nakai H, Storm TA, Kay MA. Grimm D, et al. J Virol. 2006 Jan;80(1):426-39. doi: 10.1128/JVI.80.1.426-439.2006. J Virol. 2006. PMID: 16352567 Free PMC article. - Adeno-Associated Viral Vector 2 and 9 Transduction Is Enhanced in Streptozotocin-Induced Diabetic Mouse Retina.
Lee SH, Yang JY, Madrakhimov S, Park HY, Park K, Park TK. Lee SH, et al. Mol Ther Methods Clin Dev. 2018 Dec 1;13:55-66. doi: 10.1016/j.omtm.2018.11.008. eCollection 2019 Jun 14. Mol Ther Methods Clin Dev. 2018. PMID: 30666309 Free PMC article. - Anterograde axonal transport of AAV2-GDNF in rat basal ganglia.
Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J, Bankiewicz KS. Ciesielska A, et al. Mol Ther. 2011 May;19(5):922-7. doi: 10.1038/mt.2010.248. Epub 2010 Nov 23. Mol Ther. 2011. PMID: 21102559 Free PMC article.
References
- Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12:1907-1916. - PubMed
- Arruda, V. R., P. A. Fields, R. Milner, L. Wainwright, M. P. De Miguel, P. J. Donovan, R. W. Herzog, T. C. Nichols, J. A. Biegel, M. Razavi, M. Dake, D. Huff, A. W. Flake, L. Couto, M. A. Kay, and K. High. 2001. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 4:586-592. - PubMed
- Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources