Pre-TCRalpha and TCRalpha are not interchangeable partners of TCRbeta during T lymphocyte development - PubMed (original) (raw)
Pre-TCRalpha and TCRalpha are not interchangeable partners of TCRbeta during T lymphocyte development
Christine Borowski et al. J Exp Med. 2004.
Abstract
In contrast with the alphabeta T cell receptor (TCR), the pre-TCR spontaneously segregates to membrane rafts from where it signals in a cell-autonomous fashion. The disparate behaviors of these two receptors may stem either from differences inherent to the distinct developmental stages during which they are expressed, or from features intrinsic and unique to the receptor components themselves. Here, we express TCRalpha precisely at the pre-TCR checkpoint, at levels resembling those of endogenous pre-TCRalpha (pTalpha), and in the absence of endogenous pTalpha. Both in isolation and more dramatically when in competition with pTalpha, TCRalpha induced defective proliferation, survival, and differentiation of alphabeta T lymphocyte precursors, as well as impaired commitment to the alphabeta T lymphocyte lineage. Substitution of TCRalpha transmembrane and cytoplasmic domains with those of pTalpha generated a hybrid molecule possessing enhanced competitive abilities. We conclude that features intrinsic to the pre-TCR, which are absent in TCRalpha, are essential for its unique function.
Figures
Figure 1.
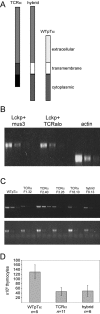
Generation of transgenic mice. (A) Structures of the WTpTα, TCRα, and TCRα/pTα hybrid molecules. (B) Semi-quantitative RT-PCR analysis of primer efficiency with β-actin control. Fivefold serial dilutions of the same sample of TCRα/pTα hybrid whole thymocyte cDNA were used in all samples. Lckp recognizes transcript p56_Lck_ proximal promoter sequences, mus3 recognizes WTpTα sequences, and TCRalo recognizes TCRα sequences. (C) Semi-quantitative RT-PCR analysis of transgene expression (top) in transgenic founders with β-actin controls (bottom). Fivefold serial dilutions of whole thymocyte cDNA were used. (D) Total thymocyte numbers in transgenic mice. Multiple founders of each transgenic construct were analyzed. Mice were analyzed at 3–5 wk of age.
Figure 2.
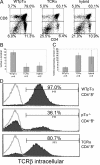
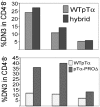
Defective differentiation, cell cycle entry, and survival of thymocytes expressing TCRα and hybrid transgenes. (A) Dot plots depict the CD4 versus CD8 profile of a minimum of five 3–5-wk-old mice of each transgenic construct. Multiple founders were analyzed. (B) Percentage of sorted Gr-1−Ter-119−Dx5−CD19−TCRγδ−CD4−CD8−CD25−CD44− (DN4) thymocytes in the S/G2/M phases of the cell cycle. DNA content was analyzed by propidium iodide staining. (C) Percentage of DN4 thymocytes expressing externalized phosphatidyl serine apoptosis marker as revealed by staining with Annexin V. (D) Percentage of CD4+8+ thymocytes expressing intracellular TCRβ (shaded histogram). Overlay represents intracellular staining with isotype-matched control antibody.
Figure 3.
Defective αβ T lineage commitment induced by TCR complexes containing TCRα and hybrid molecules. Proportion (A) and absolute number (B) of CD4−8− thymocytes expressing surface TCRγδ. (C) Percentage of CD4−8− thymocytes coexpressing TCRγδ and TCRαβ on the cell surface. (D) Histograms depict the CD4−8−TCRβ+ population representative of a minimum of five 3–5-wk-old mice of each transgenic construct. Multiple founders were analyzed. (E) Surface HSA expression on CD4−8−TCRβ+ thymocytes in TCRα (overlay) and WTpTα (fill) mice. (F) Surface HSA expression of CD4−8−TCRβ+ (shaded histogram) and CD4−8−TCRγδ+ (overlay) thymocytes in TCRα mice.
Figure 4.
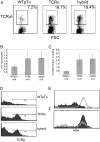
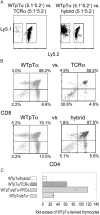
Competitive ability of TCRα, hybrid, and WTpTα-derived thymocytes. (A) Dot plots depict apparent domination of WTpTα-derived thymocytes when in direct competition with TCRα and TCRα/pTα hybrid–derived thymocytes. (B) Dot plots depict the CD4 versus CD8 surface profile of WTpTα, TCRα, and TCRα/pTα hybrid–derived thymocytes during competitive thymic reconstitution. (C) Average fold excess of WTpTα-derived thymocytes during competitive thymic reconstitution, normalized to any fold excess CD4−8−25−44+ (DN1) cells in either population. WTpTα versus _pT_α−/− competition is included as a negative control to illustrate background. Dots depict individual mice for each type of competition.
Figure 5.
Enhanced performance of hybrid at pre-TCR checkpoint requires intact pTα cytoplasmic domain. Percent of CD4−8− thymocytes expressing CD25+CD44− (DN3) surface phenotype in each competing population. Three individual mice are shown for each competition.
Figure 6.
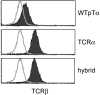
Influence of the extracellular region of TCRα on the behavior of the hybrid molecule. TCRβ levels on the surface of the 58α−β+ T cell hybridoma are influenced by the second extracellular domain TCRα. 58α−β+ hybridoma cells were infected with retroviruses encoding WTpTα, TCRα, or TCRα/pTα hybrid molecules, followed by an internal ribosomal entry site and enhanced green fluorescent protein (EGFP). Infected cells were identified by EGFP expression. Shaded histograms represent TCRβ surface staining on cells infected with retroviruses expressing WTpTα, TCRα, or TCRα/pTα hybrid molecules. The overlay represents TCRβ staining on cells infected with a control retrovirus expressing EGFP alone. The 58α−β+ T cell hybridoma is a variant of the DO-11.10.7 mouse T cell hybridoma that does not express functional TCRβ or TCRα chains (28).
Similar articles
- A critical role for the cytoplasmic tail of pTalpha in T lymphocyte development.
Aifantis I, Borowski C, Gounari F, Lacorazza HD, Nikolich-Zugich J, von Boehmer H. Aifantis I, et al. Nat Immunol. 2002 May;3(5):483-8. doi: 10.1038/ni779. Epub 2002 Apr 1. Nat Immunol. 2002. PMID: 11927911 - MHC-restricted T cell receptor signaling is required for alpha beta TCR replacement of the pre T cell receptor.
Croxford AL, Akilli-Ozturk O, Rieux-Laucat F, Förster I, Waisman A, Buch T. Croxford AL, et al. Eur J Immunol. 2008 Feb;38(2):391-9. doi: 10.1002/eji.200737054. Eur J Immunol. 2008. PMID: 18203137 - Early TCRalpha expression generates TCRalphagamma complexes that signal the DN-to-DP transition and impair development.
Erman B, Feigenbaum L, Coligan JE, Singer A. Erman B, et al. Nat Immunol. 2002 Jun;3(6):564-9. doi: 10.1038/ni800. Epub 2002 May 20. Nat Immunol. 2002. PMID: 12021779 - Structure and function of the pre-T cell receptor.
von Boehmer H, Fehling HJ. von Boehmer H, et al. Annu Rev Immunol. 1997;15:433-52. doi: 10.1146/annurev.immunol.15.1.433. Annu Rev Immunol. 1997. PMID: 9143695 Review. - Molecular basis for pre-TCR-mediated autonomous signaling.
Yamasaki S, Saito T. Yamasaki S, et al. Trends Immunol. 2007 Jan;28(1):39-43. doi: 10.1016/j.it.2006.11.006. Epub 2006 Nov 28. Trends Immunol. 2007. PMID: 17126602 Review.
Cited by
- How the TCR balances sensitivity and specificity for the recognition of self and pathogens.
Morris GP, Allen PM. Morris GP, et al. Nat Immunol. 2012 Jan 19;13(2):121-8. doi: 10.1038/ni.2190. Nat Immunol. 2012. PMID: 22261968 Review. - Human Peripheral CD4(+) Vδ1(+) γδT Cells Can Develop into αβT Cells.
Ziegler H, Welker C, Sterk M, Haarer J, Rammensee HG, Handgretinger R, Schilbach K. Ziegler H, et al. Front Immunol. 2014 Dec 17;5:645. doi: 10.3389/fimmu.2014.00645. eCollection 2014. Front Immunol. 2014. PMID: 25709606 Free PMC article. - The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor.
Maillard I, Tu L, Sambandam A, Yashiro-Ohtani Y, Millholland J, Keeshan K, Shestova O, Xu L, Bhandoola A, Pear WS. Maillard I, et al. J Exp Med. 2006 Oct 2;203(10):2239-45. doi: 10.1084/jem.20061020. Epub 2006 Sep 11. J Exp Med. 2006. PMID: 16966428 Free PMC article. - Regulation of T-cell Receptor Gene Expression by Three-Dimensional Locus Conformation and Enhancer Function.
Rodríguez-Caparrós A, Álvarez-Santiago J, Del Valle-Pastor MJ, Suñé C, López-Ros J, Hernández-Munain C. Rodríguez-Caparrós A, et al. Int J Mol Sci. 2020 Nov 11;21(22):8478. doi: 10.3390/ijms21228478. Int J Mol Sci. 2020. PMID: 33187197 Free PMC article. Review. - Cutting edge: rescue of pre-TCR but not mature TCR signaling in mice expressing membrane-targeted SLP-76.
Bezman NA, Baker RG, Lenox LE, Jordan MS, Koretzky GA. Bezman NA, et al. J Immunol. 2009 May 1;182(9):5183-7. doi: 10.4049/jimmunol.0802176. J Immunol. 2009. PMID: 19380761 Free PMC article.
References
- Irving, B.A., F.W. Alt, and N. Killeen. 1998. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 280:905–908. - PubMed
- Saint-Ruf, C., M. Panigada, O. Azogui, P. Debey, H. von Boehmer, and F. Grassi. 2000. Different initiation of pre-TCR and gammadeltaTCR signalling. Nature. 406:524–527. - PubMed
- Aifantis, I., F. Gounari, L. Scorrano, C. Borowski, and H. von Boehmer. 2001. Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nat. Immunol. 2:403–409. - PubMed
- Voll, R.E., E. Jimi, R.J. Phillips, D.F. Barber, M. Rincon, A.C. Hayday, R.A. Flavell, and S. Ghosh. 2000. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 13:677–689. - PubMed
- von Boehmer, H., I. Aifantis, J. Feinberg, O. Lechner, C. Saint-Ruf, U. Walter, J. Buer, and O. Azogui. 1999. Pleiotropic changes controlled by the pre-T-cell receptor. Curr. Opin. Immunol. 11:135–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases