Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors - PubMed (original) (raw)
. 2004 Mar 9;101(10):3662-7.
doi: 10.1073/pnas.0307231101. Epub 2004 Mar 1.
Erin M Elliott, Kong E You-Ten, Victor Y Cheng, Delia Belelli, J Glen Newell, Michael F Jackson, Jeremy J Lambert, Thomas W Rosahl, Keith A Wafford, John F MacDonald, Beverley A Orser
Affiliations
- PMID: 14993607
- PMCID: PMC373519
- DOI: 10.1073/pnas.0307231101
Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors
Valerie B Caraiscos et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The principal inhibitory neurotransmitter in the mammalian brain, gamma-aminobutyric acid (GABA), is thought to regulate memory processes by activating transient inhibitory postsynaptic currents. Here we describe a nonsynaptic, tonic form of inhibition in mouse CA1 pyramidal neurons that is generated by a distinct subpopulation of GABA type A receptors (GABA(A)Rs). This tonic inhibitory conductance is predominantly mediated by alpha5 subunit-containing GABA(A)Rs (alpha5GABA(A)Rs) that have different pharmacological and kinetic properties compared to postsynaptic receptors. GABA(A)Rs that mediate the tonic conductance are well suited to detect low, persistent, ambient concentrations of GABA in the extracellular space because they are highly sensitive to GABA and desensitize slowly. Moreover, the tonic current is highly sensitive to enhancement by amnestic drugs. Given the restricted expression of alpha5GABA(A)Rs to the hippocampus and the association between reduced alpha5GABA(A)R function and improved memory performance in behavioral studies, our results suggest that tonic inhibition mediated by alpha5GABA(A)Rs in hippocampal pyramidal neurons plays a key role in cognitive processes.
Figures
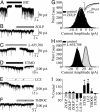
Fig. 1.
Reduced tonic current in α5-/- hippocampal slice and cultured neurons. (A) BIC (100 μM; solid bar) inhibited a tonic current recorded from CA1 pyramidal neurons in hippocampal slices from WT but not α5-/- mice. (B) Scatterplots illustrate the amplitude of the tonic current in hippocampal slices. The horizontal lines in B and D represent the mean amplitude of the tonic current. (C) Tonic current is reduced in α5-/- neurons grown in primary cultures. (D) Scatterplots illustrate the amplitude of the tonic current in cultured neurons. Inset shows that mIPSCs in α5-/- and WT neurons are similar. (E and F) The all-point histograms illustrate the amplitude of the current in the absence (black) and presence (gray) of BIC for WT and α5-/- cultured neurons. The dashed line represents the line of best fit.
Fig. 2.
Pharmacological profile of the tonic current in hippocampal neurons from Swiss White mice. Representative traces of _I_hold before and during the application of BIC (100 μM) (A), ZOLP (100 nM) (B), L-655,708 (50 μM) (C), ETMD (100 nM) (D), loreclezole (LCZ) (10 μM) (E), and allotetrahydrodeoxycorticosterone (THDOC) (10 nM) (F). (G) The corresponding all-point histograms for ZOLP treatment are shown on the right. Inset shows the averaged traces of mIPSCs recorded in the absence (black) and presence (gray) of ZOLP (100 nM). ZOLP increased the decay and charge transfer of mIPSCs. (H) The shift in the all-point histogram by L-655,708 is consistent with a reduced tonic current. (I) The bar graph summarizes the effects of drugs, including furosemide (FURO, 600 μM), on the amplitude of the tonic current. Data are presented as percent control relative to the BIC-mediated shift in current amplitude (*, P < 0.05).
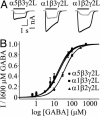
Fig. 3.
(A) Representative traces for currents evoked by 10 μM GABA (gray) and 600 μM GABA (black) from α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. The solid line denotes the duration of GABA application. (B) GABA concentration-response curves for α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. GABA sensitivity was reduced 2-fold for α1β2γ2L GABAARs compared with α5β3γ2L and α1β3γ2L GABAARs.
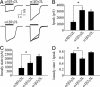
Fig. 4.
α5GABAARs expressed in human embryonic kidney 293 cells generate low-amplitude, slowly desensitizing current. (A) Representative traces of GABA-evoked currents recorded from α5β3γ2L, α1β3γ2L, and α1β2γ2L GABAARs. The solid line denotes the duration of GABA application (600 μM). Currents from α5β3γ2L and α1β3γ2L GABAARs are normalized and superimposed for illustration purposes (Lower Right). (B and C) _I_p and _I_ss are reduced for α5β3γ2L vs. α1β3γ2L. (D) The _I_ss/_I_p ratio is greater for α5β3γ2L than for α1β3γ2L GABAARs (*, P < 0.05).
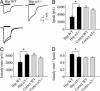
Fig. 5.
Hippocampal α5-/- neurons generate a higher-amplitude, faster-desensitizing current. (A) Representative GABA-evoked currents (600 μM) recorded from α5-/- and WT hippocampal (Hip) neurons. The solid line denotes the duration of GABA application (600 μM). The traces are normalized and superimposed for illustration purposes (Lower). (B and C) WT hippocampal neurons generate smaller _I_p and _I_ss compared with α5-/- neurons. (D) The _I_ss/_I_p ratio is increased in hippocampal WT compared with α5-/- neurons (*, P < 0.05). Note that there are no differences in _I_p, _I_ss, or _I_ss/_I_p for cortical neurons from α5-/- or WT mice.
Similar articles
- Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons.
Bonin RP, Martin LJ, MacDonald JF, Orser BA. Bonin RP, et al. J Neurophysiol. 2007 Oct;98(4):2244-54. doi: 10.1152/jn.00482.2007. Epub 2007 Aug 22. J Neurophysiol. 2007. PMID: 17715197 - alpha5 Subunit-containing GABA(A) receptors mediate a slowly decaying inhibitory synaptic current in CA1 pyramidal neurons following Schaffer collateral activation.
Vargas-Caballero M, Martin LJ, Salter MW, Orser BA, Paulsen O. Vargas-Caballero M, et al. Neuropharmacology. 2010 Mar;58(3):668-75. doi: 10.1016/j.neuropharm.2009.11.005. Epub 2009 Nov 23. Neuropharmacology. 2010. PMID: 19941877 Free PMC article. - Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice.
Glykys J, Mody I. Glykys J, et al. J Neurophysiol. 2006 May;95(5):2796-807. doi: 10.1152/jn.01122.2005. Epub 2006 Feb 1. J Neurophysiol. 2006. PMID: 16452257 - Discovering the Intriguing Properties of Extrasynaptic γ-Aminobutyric Acid Type A Receptors.
Orser BA. Orser BA. Anesthesiology. 2024 Jun 1;140(6):1192-1200. doi: 10.1097/ALN.0000000000004949. Anesthesiology. 2024. PMID: 38624275 Review. - Activation of GABAA receptors: views from outside the synaptic cleft.
Glykys J, Mody I. Glykys J, et al. Neuron. 2007 Dec 6;56(5):763-70. doi: 10.1016/j.neuron.2007.11.002. Neuron. 2007. PMID: 18054854 Review.
Cited by
- An Astrocytic Influence on Impaired Tonic Inhibition in Hippocampal CA1 Pyramidal Neurons in a Mouse Model of Rett Syndrome.
Dong Q, Kim J, Nguyen L, Bu Q, Chang Q. Dong Q, et al. J Neurosci. 2020 Aug 5;40(32):6250-6261. doi: 10.1523/JNEUROSCI.3042-19.2020. Epub 2020 Jul 2. J Neurosci. 2020. PMID: 32616668 Free PMC article. - Regulation of epileptiform activity by two distinct subtypes of extrasynaptic GABAA receptors.
Sun Y, Wu Z, Kong S, Jiang D, Pitre A, Wang Y, Chen G. Sun Y, et al. Mol Brain. 2013 May 1;6:21. doi: 10.1186/1756-6606-6-21. Mol Brain. 2013. PMID: 23634821 Free PMC article. - Potentiation of GABAA receptor activity by volatile anaesthetics is reduced by α5GABAA receptor-preferring inverse agonists.
Lecker I, Yin Y, Wang DS, Orser BA. Lecker I, et al. Br J Anaesth. 2013 Jun;110 Suppl 1(Suppl 1):i73-81. doi: 10.1093/bja/aet038. Epub 2013 Mar 27. Br J Anaesth. 2013. PMID: 23535829 Free PMC article. - Lack of an endogenous GABAA receptor-mediated tonic current in hypoglossal motoneurons.
Numata JM, van Brederode JF, Berger AJ. Numata JM, et al. J Physiol. 2012 Jul 1;590(13):2965-76. doi: 10.1113/jphysiol.2012.231944. Epub 2012 Apr 10. J Physiol. 2012. PMID: 22495589 Free PMC article. - Identification and in vitro pharmacological characterization of a novel and selective α7 nicotinic acetylcholine receptor agonist, Br-IQ17B.
Tang JS, Xie BX, Bian XL, Xue Y, Wei NN, Zhou JH, Hao YC, Li G, Zhang LR, Wang KW. Tang JS, et al. Acta Pharmacol Sin. 2015 Jul;36(7):800-12. doi: 10.1038/aps.2015.9. Epub 2015 Apr 27. Acta Pharmacol Sin. 2015. PMID: 25948478 Free PMC article.
References
- McKernan, R. M. & Whiting, P. J. (1996) Trends Neurosci. 19, 139-143. - PubMed
- Fritschy, J. M. & Mohler, H. (1995) J. Comp. Neurol. 359, 154-194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous