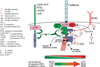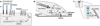Convergence of Wnt, beta-catenin, and cadherin pathways - PubMed (original) (raw)
Review
Convergence of Wnt, beta-catenin, and cadherin pathways
W James Nelson et al. Science. 2004.
Abstract
The specification and proper arrangements of new cell types during tissue differentiation require the coordinated regulation of gene expression and precise interactions between neighboring cells. Of the many growth factors involved in these events, Wnts are particularly interesting regulators, because a key component of their signaling pathway, beta-catenin, also functions as a component of the cadherin complex, which controls cell-cell adhesion and influences cell migration. Here, we assemble evidence of possible interrelations between Wnt and other growth factor signaling, beta-catenin functions, and cadherin-mediated adhesion.
Figures
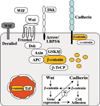
Fig. 1
The central role of β-catenin in Wnt signaling and the cadherin complex. β-Catenin exists in a cadherin-bound form that regulates adhesion; in a complex with axin, APC, and GSK-3β, where it is phosphorylated and targeted for degradation by β-TrCP; or in the nucleus with TCF/LEF transcription factors. Wnt signaling, proceeding through Frizzled and Arrow–LRP-5/6, activates Dishevelled (Dsh), which results in uncoupling β-catenin from the degradation pathway and its entry into the nucleus, where it interacts with TCF/LEF to control transcription. Wnt protein can also interact with the Derailed receptor to control axon path-finding. The Wnt pathway is also subject to extensive regulation and feedback control by extracellular factors that bind Wnt [Wnt inhibitory factor (WIF) and Frizzled-related protein (FRP)] or the coreceptor LRP (Dickkopf). The insert displays possible levels of interactions between Wnt signaling and cadherin-mediated adhesion (dotted lines) and the central role of β-catenin in both processes that are the focus of the review.
Fig. 2
Structural and functional regulation of the cadherin-catenin complex by the balance of tyrosine kinase and phosphatase activities. Cadherin binds p120 and β-catenin, which in turn binds α-catenin. The integrity of this complex is negatively regulated by phosphorylation of β-catenin by receptor tyrosine kinases (RTKs) and cytoplasmic tyrosine kinases (Fer, Fyn, Yes, and Src), which phosphorylate (red arrows) specific tyrosine residues in β-catenin (Y654, Y142), which leads to dissociation of the cadherin-catenin complex. Integrity of the cadherin-catenin complex is positively regulated by β-catenin phosphorylation by casein kinase II, and dephosphorylation by protein tyrosine phosphatases that bind p120 and β-catenin (green arrows). Changes in the phosphorylation state of β-catenin (bottom) affect cell-cell adhesion, cell migration, and the level of signaling β-catenin.
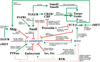
Fig. 3
Intersection of pathways controlling Wnt/β-catenin signaling and cadherin-mediated adhesion. Connections between cadherin and Wnt/β-catenin signaling pathways are based on studies in tissue culture cells and in tissues, and some involve manipulations of protein levels and expression patterns (for details, see text). All possible intersections between these pathways and their outcomes are represented together as a map, although individual pathways are likely to occur only in specific physiological contexts. Pathways that activate are indicated by solid green, pathways that reduce activity are indicated in solid red, and indirect consequences of pathway activation or inactivation are indicated by dotted lines.
Fig. 4
Additional roles of Wnt signaling components, adhesion proteins, and APC. (A) Planar polarity in Drosophila is regulated by Frizzled signaling through Dsh, the small guanosine triphosphatase (GTPase) RhoA, and its downstream effector Rho-kinase (ROCK). Although it is not known whether this process is Wnt-dependent, it is controlled by nonclassical cadherin-like adhesion molecules of the Fat-Dachsous family, as well as by Flamingo. All these molecules contain cadherin repeats, that, in the case of Flamingo, are linked to a seven-transmembrane domain. The number of cadherin repeats displayed here is arbitrary. There are numerous additional components in planar polarity not discussed here (46). (B) In addition to a role in targeting β-catenin for degradation, APC also interacts with the plus-end of microtubules at the plasma membrane of migrating cells. Recent studies indicate that APC and microtubules orient the direction of cell migration through a signaling cascade from integrins that bind extracellular matrix; the small GTPase Cdc42; the PAR complex, which contains the scaffolding proteins Par3/Par 6 and an atypical protein kinase C; and serine/threonine kinase GSK-3β (–52). (C) APC also localizes with the cadherin-catenin complex at the adherens junction (AJ), a major cell-cell adhesion complex with the tight junction (TJ) at the boundary between the apical and lateral membranes of polarized epithelial cells; E-APC may linkmicrotubules to the plasma membrane and may regulate the organization and function of the AJ (45, 53).
Similar articles
- Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk.
Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Tuli R, et al. J Biol Chem. 2003 Oct 17;278(42):41227-36. doi: 10.1074/jbc.M305312200. Epub 2003 Jul 31. J Biol Chem. 2003. PMID: 12893825 - Wnt-1 and int-2 mammary oncogene effects on the beta-catenin pathway in immortalized mouse mammary epithelial cells are not sufficient for tumorigenesis.
Hollmann CA, Kittrell FS, Medina D, Butel JS. Hollmann CA, et al. Oncogene. 2001 Nov 15;20(52):7645-57. doi: 10.1038/sj.onc.1204980. Oncogene. 2001. PMID: 11753642 - Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions.
Gottardi CJ, Gumbiner BM. Gottardi CJ, et al. Am J Physiol Cell Physiol. 2004 Apr;286(4):C747-56. doi: 10.1152/ajpcell.00433.2003. Epub 2003 Nov 12. Am J Physiol Cell Physiol. 2004. PMID: 14613891 - Cadherin-mediated cellular signaling.
Wheelock MJ, Johnson KR. Wheelock MJ, et al. Curr Opin Cell Biol. 2003 Oct;15(5):509-14. doi: 10.1016/s0955-0674(03)00101-7. Curr Opin Cell Biol. 2003. PMID: 14519384 Review. - Regulation of beta-catenin signaling in the Wnt pathway.
Kikuchi A. Kikuchi A. Biochem Biophys Res Commun. 2000 Feb 16;268(2):243-8. doi: 10.1006/bbrc.1999.1860. Biochem Biophys Res Commun. 2000. PMID: 10679188 Review.
Cited by
- Impact of laminitis on the canonical Wnt signaling pathway in basal epithelial cells of the equine digital laminae.
Wang L, Pawlak EA, Johnson PJ, Belknap JK, Eades S, Stack S, Cousin H, Black SJ. Wang L, et al. PLoS One. 2013;8(2):e56025. doi: 10.1371/journal.pone.0056025. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405249 Free PMC article. - Selective activation of p120ctn-Kaiso signaling to unlock contact inhibition of ARPE-19 cells without epithelial-mesenchymal transition.
Chen HC, Zhu YT, Chen SY, Tseng SC. Chen HC, et al. PLoS One. 2012;7(5):e36864. doi: 10.1371/journal.pone.0036864. Epub 2012 May 9. PLoS One. 2012. PMID: 22590627 Free PMC article. - Brain temperature: physiology and pathophysiology after brain injury.
Mrozek S, Vardon F, Geeraerts T. Mrozek S, et al. Anesthesiol Res Pract. 2012;2012:989487. doi: 10.1155/2012/989487. Epub 2012 Dec 26. Anesthesiol Res Pract. 2012. PMID: 23326261 Free PMC article. - Human telomerase reverse transcriptase (hTERT) is a target gene of β-catenin in human colorectal tumors.
Jaitner S, Reiche JA, Schäffauer AJ, Hiendlmeyer E, Herbst H, Brabletz T, Kirchner T, Jung A. Jaitner S, et al. Cell Cycle. 2012 Sep 1;11(17):3331-8. doi: 10.4161/cc.21790. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22894902 Free PMC article. - The ER membrane protein complex subunit Emc3 controls angiogenesis via the FZD4/WNT signaling axis.
Yang M, Li S, Liu W, Li X, He Y, Yang Y, Sun K, Zhang L, Tian W, Duan L, Chen H, Yao D, Yang Z, Zhu X. Yang M, et al. Sci China Life Sci. 2021 Nov;64(11):1868-1883. doi: 10.1007/s11427-021-1941-7. Epub 2021 Jun 10. Sci China Life Sci. 2021. PMID: 34128175
References
- Affolter M, et al. Dev. Cell. 2003;4:11. - PubMed
- Cadigan K, Nusse R. Genes Dev. 1997;11:3286. - PubMed
- Thiery JP. Nature Rev. Cancer. 2002;2:442. - PubMed
- Massague J, Blain SW, Lo RS. Cell. 2000;103:295. - PubMed
- Jamora C, Fuchs E. Nature Cell Biol. 2002;4:E101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous