Febrigenic signaling to the brain does not involve nitric oxide - PubMed (original) (raw)
Comparative Study
. 2004 Apr;141(7):1204-13.
doi: 10.1038/sj.bjp.0705713. Epub 2004 Mar 8.
Affiliations
- PMID: 15006900
- PMCID: PMC1574882
- DOI: 10.1038/sj.bjp.0705713
Comparative Study
Febrigenic signaling to the brain does not involve nitric oxide
Alexandre A Steiner et al. Br J Pharmacol. 2004 Apr.
Abstract
1. The involvement of peripheral nitric oxide (NO) in febrigenic signaling to the brain has been proposed because peripherally administered NO synthase (NOS) inhibitors attenuate lipopolysaccharide (LPS)-induced fever in rodents. However, how the unstable molecule of NO can reach the brain to trigger fever is unclear. It is also unclear whether NOS inhibitors attenuate fever by blocking febrigenic signaling or, alternatively, by suppressing thermogenesis in brown fat. 2. Male Wistar rats were chronically implanted with jugular catheters; their colonic and tail skin temperatures (T(c) and T(sk)) were monitored. 3. Study 1 was designed to determine whether the relatively stable, physiologically relevant forms of NO, that is, S-nitrosoalbumin (SNA) and S-nitrosoglutathione (SNG), are pyrogenic and whether they enhance LPS fever. At a neutral ambient temperature (T(a)) of 31 degrees C, afebrile or LPS (1 microg kg(-1), i.v.)-treated rats were infused i.v. with SNA (0.34 or 4.1 micromol kg(-1); the controls received NaNO(2) and albumin) or SNG (10 or 60 micromol kg(-1); the controls received glutathione). T(c) of SNA- or SNG-treated rats never exceeded that of the controls. 4. In Study 2, we tested whether the known fever-attenuating effect of the NOS inhibitor N(omega)-nitro-L-arginine methyl ester (L-NAME) at a subneutral T(a) (when fever is brought about by thermogenesis) also occurs at a neutral T(a) (when fever is brought about by skin vasoconstriction). At a subneutral T(a) of 24 degrees C, L-NAME (2.5 mg kg(-1), i.v.) attenuated LPS (10 microg kg(-1), i.v.) fever, presumably by inhibiting thermogenesis. At 31 degrees C, L-NAME enhanced LPS fever by augmenting skin vasoconstriction (T(sk) fall). 5. In summary, both SNA and SNG had no pyrogenic effect of their own and failed to enhance LPS fever; peripheral L-NAME attenuated only fever brought about by increased thermogenesis. It is concluded that NO is uninvolved in febrigenic signaling to the brain.
Figures
Figure 1
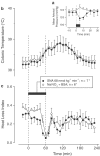
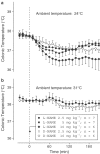
Effects of SNA (infusion rate indicated) on the MAP (a), colonic temperature (b), and HLI (c) of afebrile rats at a neutral ambient temperature of 31°C. The controls received the corresponding amounts of NaNO2 and BSA; see Methods for details. The black bars indicate the duration of infusions. *In panel (a), _n_=3 for each group.
Figure 2
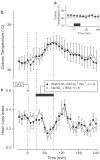
Effects of SNG (infusion rate indicated) on the MAP (a), colonic temperature (b), and HLI (c) of afebrile rats at 31°C. The controls received reduced glutathione. The black bars indicate the duration of infusions. *In panel (a), _n_=3 for each group.
Figure 3
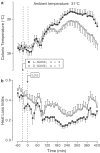
Effects of SNA (infusion rate indicated) on the colonic temperature of afebrile rats (a) and on the colonic temperature (b) and HLI (c) of LPS (1 _μ_g kg−1, i.v.)-treated rats at 31°C. The controls were infused with the corresponding amounts of NaNO2 and BSA. The black bars indicate the duration of infusions.
Figure 4
Effects of SNG (infusion rate indicated) on the colonic temperature of afebrile rats (a) and on the colonic temperature (b) and HLI (c) of LPS (1 _μ_g kg−1, i.v.)-treated rats at 31°C. The controls received reduced glutathione. The black bars indicate the duration of infusions.
Figure 5
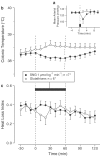
Effect of intravenous injection of the NOS inhibitor
L
-NAME (doses indicated) or its inactive enantiomer
D
-NAME (doses indicated) on the colonic temperature of rats at an ambient temperature of 24°C (a) or 31°C (b).
Figure 6
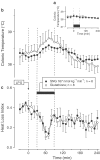
Effect of pretreatment with
L
-NAME (2.5 mg kg−1, i.v.) or
D
-NAME on the colonic temperature (a) and HLI (b) of LPS (10 _μ_g kg−1, i.v.)-treated rats exposed to an ambient temperature of 24°C.
Figure 7
Effect of
L
-NAME (2.5 mg kg−1, i.v.) or
D
-NAME on the colonic temperature (a) and HLI (b) of LPS (10 mg kg−1, i.v.)-treated rats exposed to an ambient temperature of 31°C.
Similar articles
- Nitric oxide and fever: immune-to-brain signaling vs. thermogenesis in chicks.
Dantonio V, Batalhão ME, Fernandes MH, Komegae EN, Buqui GA, Lopes NP, Gargaglioni LH, Carnio ÉC, Steiner AA, Bícego KC. Dantonio V, et al. Am J Physiol Regul Integr Comp Physiol. 2016 May 15;310(10):R896-905. doi: 10.1152/ajpregu.00453.2015. Epub 2016 Mar 16. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26984892 - Evidence that inducible nitric oxide synthase is involved in LPS-induced plasma leakage in rat skin through the activation of nuclear factor-kappaB.
Iuvone T, D'Acquisto F, Van Osselaer N, Di Rosa M, Carnuccio R, Herman AG. Iuvone T, et al. Br J Pharmacol. 1998 Apr;123(7):1325-30. doi: 10.1038/sj.bjp.0701730. Br J Pharmacol. 1998. PMID: 9579726 Free PMC article. - Blockade of nitric oxide formation in the rat brain does not disturb development of endotoxin tolerance.
Soszynski D, Daniluk M, Galazka M, Dmitruk K. Soszynski D, et al. J Physiol Pharmacol. 2013 Dec;64(6):779-88. J Physiol Pharmacol. 2013. PMID: 24388893 - Attenuation by nitrosothiol NO donors of acute intestinal microvascular dysfunction in the rat.
László F, Whittle BJ, Moncada S. László F, et al. Br J Pharmacol. 1995 Jun;115(3):498-502. doi: 10.1111/j.1476-5381.1995.tb16361.x. Br J Pharmacol. 1995. PMID: 7582463 Free PMC article. - Nitric oxide pathway is an important modulator of heat loss in rats during exercise.
Lacerda AC, Marubayashi U, Coimbra CC. Lacerda AC, et al. Brain Res Bull. 2005 Sep 30;67(1-2):110-6. doi: 10.1016/j.brainresbull.2005.06.002. Brain Res Bull. 2005. PMID: 16140169
Cited by
- Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor.
Dogan MD, Patel S, Rudaya AY, Steiner AA, Székely M, Romanovsky AA. Dogan MD, et al. Br J Pharmacol. 2004 Dec;143(8):1023-32. doi: 10.1038/sj.bjp.0705977. Epub 2004 Oct 18. Br J Pharmacol. 2004. PMID: 15492017 Free PMC article. - A review of the physiology of fever in birds.
Gray DA, Marais M, Maloney SK. Gray DA, et al. J Comp Physiol B. 2013 Apr;183(3):297-312. doi: 10.1007/s00360-012-0718-z. Epub 2012 Nov 18. J Comp Physiol B. 2013. PMID: 23160839 Review. - Regulation of MyD88-dependent signaling events by S nitrosylation retards toll-like receptor signal transduction and initiation of acute-phase immune responses.
Into T, Inomata M, Nakashima M, Shibata K, Häcker H, Matsushita K. Into T, et al. Mol Cell Biol. 2008 Feb;28(4):1338-47. doi: 10.1128/MCB.01412-07. Epub 2007 Dec 17. Mol Cell Biol. 2008. PMID: 18086890 Free PMC article. - Hyperbilirubinemia exaggerates endotoxin-induced hypothermia.
Pakai E, Garami A, Nucci TB, Ivanov AI, Romanovsky AA. Pakai E, et al. Cell Cycle. 2015;14(8):1260-7. doi: 10.1080/15384101.2015.1014150. Cell Cycle. 2015. PMID: 25774749 Free PMC article. - Leptin: at the crossroads of energy balance and systemic inflammation.
Steiner AA, Romanovsky AA. Steiner AA, et al. Prog Lipid Res. 2007 Mar;46(2):89-107. doi: 10.1016/j.plipres.2006.11.001. Epub 2006 Dec 21. Prog Lipid Res. 2007. PMID: 17275915 Free PMC article. Review.
References
- ATAOGLU H., DOGAN M.D., MUSTAFA F., AKARSU E.S. Candida albicans and Saccharomyces cerevisiae cell wall mannans produce fever in rats: role of nitric oxide and cytokines. Life Sci. 2000;67:2247–2256. - PubMed
- BRANCO L.G.S., CARNIO E.C., BARROS R.C.H. Role of nitric oxide pathway in hypoxia-induced hypothermia of rats. Am. J. Physiol. 1997;273:R967–R971. - PubMed
- CLAY K.L., JOHNSON C., HENSON P. Binding of platelet activating factor to albumin. Biochim. Biophys. Acta. 1990;1046:309–314. - PubMed
- CONDORELLI P., GEORGE S.C. Free nitric oxide diffusion in the bronchial microcirculation. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H2660–H2670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials