Myosin V: regulation by calcium, calmodulin, and the tail domain - PubMed (original) (raw)
Myosin V: regulation by calcium, calmodulin, and the tail domain
Dimitry N Krementsov et al. J Cell Biol. 2004.
Abstract
Calcium activates the ATPase activity of tissue-purified myosin V, but not that of shorter expressed constructs. Here, we resolve this discrepancy by comparing an expressed full-length myosin V (dFull) to three shorter constructs. Only dFull has low ATPase activity in EGTA, and significantly higher activity in calcium. Based on hydrodynamic data and electron microscopic images, the inhibited state is due to a compact conformation that is possible only with the whole molecule. The paradoxical finding that dFull moved actin in EGTA suggests that binding of the molecule to the substratum turns it on, perhaps mimicking cargo activation. Calcium slows, but does not stop the rate of actin movement if excess calmodulin (CaM) is present. Without excess CaM, calcium binding to the high affinity sites dissociates CaM and stops motility. We propose that a folded-to-extended conformational change that is controlled by calcium and CaM, and probably by cargo binding itself, regulates myosin V's ability to transport cargo in the cell.
Figures
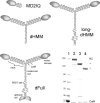
Figure 1.
Myosin V constructs. Schematic representations of MD2IQ, dHMM, long-dHMM, and the full-length construct, dFull. (Bottom right) SDS-PAGE of mol wt standards (kD, lane 1) and the purified expressed proteins: dFull (lane 2), dHMM (lane 3), and MD2IQ (lane 4).
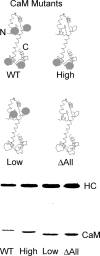
Figure 2.
Schematic diagram of CaM mutants and coexpression of CaM mutants with the MD2IQ HC. (Top) WT-CaM has four calcium-binding sites (gray spheres). Mutagenesis was used to render either the NH2-terminal low affinity, the COOH-terminal high affinity, or all the calcium-binding sites nonfunctional. The resulting mutants are called CaM-high (containing only the high affinity sites), CaM-low (containing only the low affinity sites), or CaM Δall, which has no functional calcium-binding sites. (Bottom) SDS gel of MD2IQ coexpressed with WT or mutant CaMs. The protein samples contained EGTA. In the presence of calcium, the mobility of WT-CaM and CaM-high increases, whereas that of CaM-low or CaM Δall does not (not depicted).
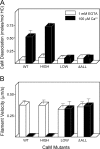
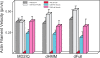
Figure 3.
High affinity calcium-binding sites are responsible for calcium-dependent CaM dissociation and motility inhibition of MD2IQ. (A) CaM dissociation from MD2IQ (0.4 mg/ml) coexpressed with various CaM mutants. CaM dissociation was determined using the actin-pelleting assay (see Materials and methods). (B) Actin filament velocity supported by MD2IQ coexpressed with the same CaM mutants. (White bars) 1 mM EGTA; (black bars) 100 μM free Ca2+.
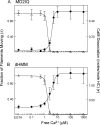
Figure 4.
Calcium dependence of CaM dissociation and motility inhibition. (A) MD2IQ. (B) dHMM. Fraction of actin filaments moving in a motility assay (open triangles, normalized to the value in EGTA) and amount of CaM dissociation (solid circles) as a function of calcium concentration. To determine the amount of CaM dissociation, samples were dialyzed into buffers containing the desired amount of free Ca2+ before pelleting with actin (see Materials and methods). The same buffers were used for in vitro motility at that free Ca2+ concentration.
Figure 5.
Rescue of motility in calcium by exogenous CaM for MD2IQ, dHMM, and dFull. Actin filament velocity by three different constructs in EGTA (dark gray bars), in EGTA with 12 μM free WT-CaM (light gray bars). 100 μM free Ca2+ completely inhibited motility (red bars), which was partially restored in 100 μM free Ca2+ and 12 μM free WT-CaM (blue bars), or more fully restored in 100 μM free Ca2+ and 12 μM CaMΔall (pink bars).
Figure 6.
Calcium dependence of the actin-activated ATPase activity in the presence of exogenous CaM. (A) MD2IQ, (B) dHMM, (C) long-dHMM, (D) dFull. 1 mM EGTA + 6 μM CaM (open circles), 100 μM free Ca2+ and 6 μM CaM (solid circles). Representative experiments are shown, which gave the following Vmax and Km values, respectively: MD2IQ (22.3 s−1 and 8.1 μM in EGTA, 23.0 s−1 and 11 μM in Ca2+); dHMM (12.8 s−1 and 1.6 μM in EGTA, 18 s−1 and 1.9 μM in Ca2+); long-dHMM (10.3 s−1 and 1.8 μM in EGTA, 16.0 s−1 and 1.0 μM in Ca2+); dFull (2.2 s−1 and 5.1 μM in EGTA, 14.8 s−1 and 1.3 μM in Ca2+). Table I summarizes the Vmax and Km values for multiple preparations.
Figure 7.
A salt- or calcium-induced conformational change in full-length myosin V as shown by sedimentation velocity. (A–C) Absorbance as a function of distance and time; (C–E) the sedimentation coefficients determined from these data by curve fitting to one species, using the dc/dt program (Philo, 2000). In 0.1 M NaCl and 1 mM EGTA (A and D) a folded, inactive myosin V sediments at 13.9 S. With increased salt concentration (B and E), the value decreases to 9.7 S indicating a more extended conformation (0.3 M NaCl). Calcium induces a similar active and extended conformation (10.8 S) near physiological ionic strength (0.1 M NaCl, with added CaM to prevent dissociation). Table II summarizes data from multiple experiments.
Figure 8.
Metal-shadowed images of full-length myosin V. Myosin V in (A) an extended or (B) more compact conformation. Yellow arrowheads point to the globular tail, and blue arrowheads to the head–rod junction. Note that the length of the tail is shorter in the compact conformation. The molecules shown in A were diluted into high ionic strength for rotary shadowing, whereas those in B were obtained in a lower ionic strength buffer. In rows 1 and 3, the molecules are oriented with the heads up and the tail down. Bar, 30 nm.
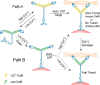
Figure 9.
A model for calcium regulation of myosin V in vivo. Two possible pathways of activation of myosin V are shown. Path A: myosin V activation via calcium, by a change from an inactive (compact) to active (extended) conformation. After binding of ATP, actin, and cargo (not necessarily in that order), transport is initiated, provided that CaM is in excess. If CaM is not in excess, motility will probably cease. A subsequent decrease in calcium concentration causes faster myosin V transport. Path B: a hypothetical pathway whereby cargo binding causes unfolding and activation of myosin V at low calcium. Once activated, the velocity of transport varies depending on the calcium and CaM concentrations.
Similar articles
- Ca2+-induced activation of ATPase activity of myosin Va is accompanied with a large conformational change.
Li XD, Mabuchi K, Ikebe R, Ikebe M. Li XD, et al. Biochem Biophys Res Commun. 2004 Mar 12;315(3):538-45. doi: 10.1016/j.bbrc.2004.01.084. Biochem Biophys Res Commun. 2004. PMID: 14975734 - Regulation of myosin V processivity by calcium at the single molecule level.
Lu H, Krementsova EB, Trybus KM. Lu H, et al. J Biol Chem. 2006 Oct 20;281(42):31987-94. doi: 10.1074/jbc.M605181200. Epub 2006 Aug 18. J Biol Chem. 2006. PMID: 16920704 - Regulation and recycling of myosin V.
Taylor KA. Taylor KA. Curr Opin Cell Biol. 2007 Feb;19(1):67-74. doi: 10.1016/j.ceb.2006.12.014. Epub 2007 Jan 8. Curr Opin Cell Biol. 2007. PMID: 17208425 Review. - Calcium-induced quenching of intrinsic fluorescence in brain myosin V is linked to dissociation of calmodulin light chains.
Cameron LC, Carvalho RN, Araujo JR, Santos AC, Tauhata SB, Larson RE, Sorenson MM. Cameron LC, et al. Arch Biochem Biophys. 1998 Jul 1;355(1):35-42. doi: 10.1006/abbi.1998.0700. Arch Biochem Biophys. 1998. PMID: 9647664 - Myosin V motor proteins: marching stepwise towards a mechanism.
Vale RD. Vale RD. J Cell Biol. 2003 Nov 10;163(3):445-50. doi: 10.1083/jcb.200308093. J Cell Biol. 2003. PMID: 14610051 Free PMC article. Review.
Cited by
- Mitochondria dynamism: of shape, transport and cell migration.
da Silva AF, Mariotti FR, Máximo V, Campello S. da Silva AF, et al. Cell Mol Life Sci. 2014 Jun;71(12):2313-24. doi: 10.1007/s00018-014-1557-8. Epub 2014 Jan 18. Cell Mol Life Sci. 2014. PMID: 24442478 Free PMC article. Review. - Random walk of processive, quantum dot-labeled myosin Va molecules within the actin cortex of COS-7 cells.
Nelson SR, Ali MY, Trybus KM, Warshaw DM. Nelson SR, et al. Biophys J. 2009 Jul 22;97(2):509-18. doi: 10.1016/j.bpj.2009.04.052. Biophys J. 2009. PMID: 19619465 Free PMC article. - The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis.
MacDougall DD, Lin Z, Chon NL, Jackman SL, Lin H, Knight JD, Anantharam A. MacDougall DD, et al. J Gen Physiol. 2018 Jun 4;150(6):783-807. doi: 10.1085/jgp.201711944. Epub 2018 May 24. J Gen Physiol. 2018. PMID: 29794152 Free PMC article. Review. - Autoinhibition and activation of myosin VI revealed by its cryo-EM structure.
Niu F, Li L, Wang L, Xiao J, Xu S, Liu Y, Lin L, Yu C, Wei Z. Niu F, et al. Nat Commun. 2024 Feb 8;15(1):1187. doi: 10.1038/s41467-024-45424-7. Nat Commun. 2024. PMID: 38331992 Free PMC article. - Simultaneous observation of tail and head movements of myosin V during processive motion.
Lu H, Kennedy GG, Warshaw DM, Trybus KM. Lu H, et al. J Biol Chem. 2010 Dec 31;285(53):42068-74. doi: 10.1074/jbc.M110.180265. Epub 2010 Oct 25. J Biol Chem. 2010. PMID: 20974847 Free PMC article.
References
- Beckingham, K. 1991. Use of site-directed mutations in the individual Ca2+-binding sites of calmodulin to examine Ca2+-induced conformational changes. J. Biol. Chem. 266:6027–6030. - PubMed
- Casaletti, L., S.B. Tauhata, J.E. Moreira, and R.E. Larson. 2003. Myosin-Va proteolysis by Ca2+/calpain in depolarized nerve endings from rat brain. Biochem. Biophys. Res. Commun. 308:159–164. - PubMed
- Cheney, R.E., M.K. O'Shea, J.E. Heuser, M.V. Coelho, J.S. Wolenski, E.M. Espreafico, P. Forscher, R.E. Larson, and M.S. Mooseker. 1993. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 75:13–23. - PubMed
- Coy, D.L., W.O. Hancock, M. Wagenbach, and J. Howard. 1999. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nat. Cell Biol. 1:288–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases