Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States - PubMed (original) (raw)
Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States
Edward Nwanegbo et al. Clin Diagn Lab Immunol. 2004 Mar.
Abstract
One of the major limitations of the use of adenoviruses as gene therapy vectors is the existence of preformed immunity in various populations. Recent studies have linked failure of adenoviral gene therapy trials to the presence of antiadenoviral neutralizing antibodies (NAb). Understanding the distribution and specificity of such antibodies will assist in the design of successful recombinant adenoviral gene therapies and vaccines. To assess the prevalence of NAb to adenovirus serotypes 5 and 35 (Ad5 and Ad35), we analyzed serum samples from adult immunocompetent individuals living in The Gambia, South Africa, and the United States by using a neutralization assay. Serum samples were incubated with A549 lung carcinoma cells and adenoviruses encoding enhanced green or yellow fluorescent proteins; results were analyzed by fluorescence microscopy and flow cytometry. Using this technique, we found a high prevalence of NAb against Ad5 in Gambian, South African, and U.S. subjects at both low and high titers. Conversely, all subjects displayed a low prevalence of NAb to Ad35; when present, anti-Ad35 NAb were seen at low titers. Because of the ability of adenoviruses to elicit systemic and mucosal immune responses, Ad35 with its low NAb prevalence appears to be an attractive candidate vector for gene therapy applications.
Figures
FIG. 1.
Infection of A549 cells by recombinant adenoviruses at various Vp/c ratios in the presence of serum. (A) Infectivity profile of Ad5EGFP in the presence of diluted serum containing NAb indicates that a Vp/c ratio of 1,000 (or multiplicity of infection [MOI] of 10) yields optimal cell infection, with a clear inverse relationship between cell infection and serum NAb concentration. At Vp/c = 100 (or MOI = 1), the percentage of infected cells did not change with decreasing serum concentration (increasing titer), whereas at Vp/c = 1,000, cell infection increased with decreased serum concentration. Infection at higher viral doses suggested viral saturation (data not shown). (B) Infection of A549 cells with Ad35EYFP reveals a similar profile as with Ad5EGFP, with Vp/c = 1,000 as the optimal viral dose.
FIG. 2.
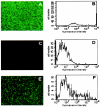
Fluorescence microscopy and flow cytometry of A549 cells after neutralization assay. (A and B) A549 cells infected with virus Ad35EYFP in the absence of serum exhibit marked fluorescence, as seen in the micrograph (A) and the histogram (B). Similar results were seen in cells infected with Ad5EGFP. (C and D) A549 cells incubated with DMEM but not infected with virus display almost no background fluorescence in either the micrograph (C) or the histogram (D). (E and F) Representative fluorescence micrograph (E) and histogram (F) demonstrating A549 cells incubated with a test serum sample and virus.
FIG. 3.
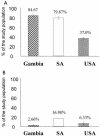
Prevalence of anti-Ad5 and anti-Ad35 NAb in the African and North American populations. (A) Anti-Ad5 NAb is present in The Gambian, South African, and U.S. populations at 84.67, 79.87, and 37.0% prevalence, respectively. (B) There is a very low prevalence of anti-Ad35 NAb in all three populations (<20%).
Similar articles
- Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa.
Thorner AR, Vogels R, Kaspers J, Weverling GJ, Holterman L, Lemckert AA, Dilraj A, McNally LM, Jeena PM, Jepsen S, Abbink P, Nanda A, Swanson PE, Bates AT, O'Brien KL, Havenga MJ, Goudsmit J, Barouch DH. Thorner AR, et al. J Clin Microbiol. 2006 Oct;44(10):3781-3. doi: 10.1128/JCM.01249-06. J Clin Microbiol. 2006. PMID: 17021110 Free PMC article. - International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations.
Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng'ang'a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. Barouch DH, et al. Vaccine. 2011 Jul 18;29(32):5203-9. doi: 10.1016/j.vaccine.2011.05.025. Epub 2011 May 25. Vaccine. 2011. PMID: 21619905 Free PMC article. - International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials.
Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. Mast TC, et al. Vaccine. 2010 Jan 22;28(4):950-7. doi: 10.1016/j.vaccine.2009.10.145. Epub 2009 Nov 17. Vaccine. 2010. PMID: 19925902 - Seroprevalence of neutralizing antibodies against adenovirus type 14 and 55 in healthy adults in Southern China.
Zheng X, Rong X, Feng Y, Sun X, Li L, Wang Q, Wang M, Liu W, Li C, Yang Y, Zhou R, Lu J, Feng L, Chen L. Zheng X, et al. Emerg Microbes Infect. 2017 Jun 7;6(6):e43. doi: 10.1038/emi.2017.29. Emerg Microbes Infect. 2017. PMID: 28588291 Free PMC article. - Global Prevalence of Preexisting Antibodies against Human Adenoviruses, Surveyed from 1962 to 2021.
Luo H, Zhou Q, Feng J, Wu Y, Chen H, Mao M, Qi R. Luo H, et al. Intervirology. 2024;67(1):19-39. doi: 10.1159/000538233. Epub 2024 Mar 8. Intervirology. 2024. PMID: 38452738 Free PMC article. Review.
Cited by
- Human Adenovirus Serotype 3 Vector Packaged by a Rare Serotype 14 Hexon.
Su X, Tian X, Jiang Z, Ma Q, Liu Q, Lu X, Zhou R. Su X, et al. PLoS One. 2016 Jun 21;11(6):e0156984. doi: 10.1371/journal.pone.0156984. eCollection 2016. PLoS One. 2016. PMID: 27328032 Free PMC article. - Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway.
Leopold PL, Wendland RL, Vincent T, Crystal RG. Leopold PL, et al. J Virol. 2006 Oct;80(20):10237-47. doi: 10.1128/JVI.00512-06. J Virol. 2006. PMID: 17005701 Free PMC article. - Translational mini-review series on vaccines: Development and evaluation of improved vaccines against tuberculosis.
Sander C, McShane H. Sander C, et al. Clin Exp Immunol. 2007 Mar;147(3):401-11. doi: 10.1111/j.1365-2249.2006.03306.x. Clin Exp Immunol. 2007. PMID: 17302888 Free PMC article. Review. - Oncolytic Viruses and Cancer, Do You Know the Main Mechanism?
Kooti W, Esmaeili Gouvarchin Ghaleh H, Farzanehpour M, Dorostkar R, Jalali Kondori B, Bolandian M. Kooti W, et al. Front Oncol. 2021 Dec 22;11:761015. doi: 10.3389/fonc.2021.761015. eCollection 2021. Front Oncol. 2021. PMID: 35004284 Free PMC article. Review. - Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients.
Butterfield LH, Economou JS, Gamblin TC, Geller DA. Butterfield LH, et al. J Transl Med. 2014 Apr 5;12:86. doi: 10.1186/1479-5876-12-86. J Transl Med. 2014. PMID: 24708667 Free PMC article.
References
- Brody, S. L., M. Metzger, C. Danel, M. A. Rosenfeld, and R. G. Crystal. 1994. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 5:821-836. - PubMed
- Chen, Y., D.-C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existing adenovirus antibody inhibits systemic toxicity and anti-tumour activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposal for human therapy. Hum. Gene Ther. 11:1553-1567. - PubMed
- De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. V. Slaterus, G. J. van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenovirus from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources