An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A - PubMed (original) (raw)
. 2004 Apr 7;23(7):1506-15.
doi: 10.1038/sj.emboj.7600164. Epub 2004 Mar 11.
Hyewon Shin, Jeonghoon Choi, Jaewon Ko, Seho Kim, Hyun Woo Lee, Karam Kim, Seong-Hwan Rho, Jun Hyuck Lee, Hye-Eun Song, Soo Hyun Eom, Eunjoon Kim
Affiliations
- PMID: 15014437
- PMCID: PMC391070
- DOI: 10.1038/sj.emboj.7600164
An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A
Jae-Ran Lee et al. EMBO J. 2004.
Abstract
Motor proteins not actively involved in transporting cargoes should remain inactive at sites of cargo loading to save energy and remain available for loading. KIF1A/Unc104 is a monomeric kinesin known to dimerize into a processive motor at high protein concentrations. However, the molecular mechanisms underlying monomer stabilization and monomer-to-dimer transition are not well understood. Here, we report an intramolecular interaction in KIF1A between the forkhead-associated (FHA) domain and a coiled-coil domain (CC2) immediately following the FHA domain. Disrupting this interaction by point mutations in the FHA or CC2 domains leads to a dramatic accumulation of KIF1A in the periphery of living cultured neurons and an enhancement of the microtubule (MT) binding and self-multimerization of KIF1A. In addition, point mutations causing rigidity in the predicted flexible hinge disrupt the intramolecular FHA-CC2 interaction and increase MT binding and peripheral accumulation of KIF1A. These results suggest that the intramolecular FHA-CC2 interaction negatively regulates KIF1A activity by inhibiting MT binding and dimerization of KIF1A, and point to a novel role of the FHA domain in the regulation of kinesin motors.
Figures
Figure 1
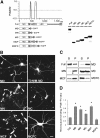
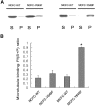
The CC2 domain negatively regulates KIF1A. (A) A schematic showing the coiled-coil probability of KIF1A. EGFP-KIF1A deletion variants are shown along with their expression levels in HEK293T cells. MD, motor domain; N, neck CC; C, CC; F, FHA domain; LBD, liprin-α-binding domain (Shin et al, 2003); P, PH domain. The schematic shows only the two most prominent coiled coils of KIF1A. Note that relatively weak coiled coils, including the neck coiled coil (aa 366–397) and one near aa 800, are invisible and weakly visible, respectively, in this high-stringency prediction. (B) Subcellular distribution pattern of KIF1A deletions in living neurons. Cultured hippocampal neurons at days in vitro (DIV) 14–18 were transfected with EGFP-KIF1A deletions and visualized 48–72 h after transfection. MD, MN, MC, and MCF accumulated in the periphery of neurons (arrowheads), whereas MD-T312M and MCFC were diffusely distributed. Scale bar: 10 μm. (C) MT-binding properties of the KIF1A deletions. Lysates of HEK293T cells transfected with KIF1A deletions were incubated with taxol-stabilized MTs, and the sedimented (P) KIF1A proteins, along with those in the supernatant (S), were analyzed by immunoblotting with EGFP antibodies. (D) Quantitative analysis of the MT binding (mean±s.d.) of KIF1A deletions. Significant increases (*, compared to Full) are indicated.
Figure 2
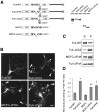
The CC2 and FHA domains are required for the negative regulation of KIF1A. (A) A schematic representation of the deletion variants of KIF1A-Full and MCFC, and their expression levels in heterologous (HEK293T) cells. Full-WT, full-length wild-type KIF1A. (B) Subcellular distribution of KIF1A deletions in living cultured hippocampal neurons. Scale bar: 10 μm. (C) MT binding of the KIF1A deletion variants. (D) Quantitative analysis of the MT binding of KIF1A deletions. Significant increases (*, compared to Full-WT; **, compared to MCFC) are indicated.
Figure 3
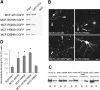
The FHA domain of KIF1A interacts with CC2. (A–D) Pull-down of KIF1A deletions. EGFP-KIF1A deletions expressed in HEK293T cells were incubated with GST–CC2 (A, B), GST–FHA (C, D), or GST alone (A–D), and the precipitates were analyzed by immunoblotting with EGFP antibodies.
Figure 4
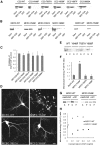
Disrupting the FHA–CC2 interaction by point mutations in the FHA domain increases KIF1A activity. (A) GST–CC2 pull-down of MCF with point mutations in the FHA domain. (B) Subcellular distribution of MCFC-EGFP containing FHA point mutations in living cultured hippocampal neurons. Scale bar: 10 μm. (C) MT binding of MCFC containing FHA point mutations. (D) Quantitative analysis of MT binding of the MCFC mutants. Significant increases (*, compared to MCFC-WT) are indicated.
Figure 5
Disrupting the FHA–CC2 interaction by point mutations in CC2 increases KIF1A activity. (A) GST–FHA pull-down of EGFP–CC2 with point mutations. (B) Pull-down of MCFC-EGFP containing point mutations in CC2 with GST–CC2, GST–FHA, or GST alone. (C) Quantitative analysis of the pull-down of mutated EGFP–CC2 with GST–CC2. (D) Subcellular distribution of MCFCs with CC2 point mutations in living cultured hippocampal neurons. Scale bar: 10 μm. (E, F) MT binding of MCFC-EGFP mutants, and quantitative analysis of the MT-binding results. A significant increase (*, compared to MCFC-WT) is indicated. (G) MT binding of MCFC-EGFP (wild type and Y649F) in the presence of AMP-PNP or ATP. (H) MT binding of MCFC-EGFP (wild type and Y649F) at various MT concentrations.
Figure 6
Enhanced MT binding of KIF1A induced by the disruption of the FHA–CC2 interaction occurs through the motor domain. (A) MT binding of EGFP-NCFC (wild type and Y649F) and MCFC-EGFP (wild type and Y649F). (B) Quantitative analysis of the results. A significant increase (*, compared to wild type) is indicated.
Figure 7
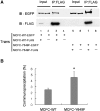
Disruption of the FHA–CC2 interaction enhances KIF1A multimerization. (A) Enhanced self-multimerization in MCFC by the introduction of the Y649F mutation. To compare self-multimerization in wild-type and mutant (Y649F) MCFC, HEK293T cells were doubly transfected with MCFC constructs differentially tagged with EGFP and FLAG (MCFC-wild-type-EGFP+MCFC-wild-type-FLAG; lanes 1–4, or MCFC-Y649F-EGFP+MCFC-Y649F-FLAG; lanes 5–8). Cell lysates were immunoprecipitated with FLAG antibodies and characterized by immunoblotting with EGFP and FLAG antibodies. Cells singly transfected with MCFC-wild-type-EGFP or MCFC-Y649F-EGFP were used as controls. FLAG-tagged MCFCs (wild-type and Y649F) are visible in self-immunoprecipitation lanes (lanes 3,7) but not in input lanes (2.5%; lanes 1,5), perhaps due to a relatively low sensitivity of the FLAG antibody. Note that the coimmunoprecipitation level of MCFC-EGFP was increased by the mutation. (B) Quantitative analysis of the coimmunoprecipitation results. A significant increase (*, compared to MCFC-WT) is indicated.
Figure 8
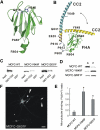
A flexible hinge in CC2 allows the intramolecular FHA–CC2 interaction. (A) A ribbon diagram of the FHA domain of KIF1A based upon homology modeling. P488 and R604 indicate the residues at the N- and C-terminal ends of the FHA domain, respectively. G519 and H540 (purple) are two residues critical for the FHA–CC2 interaction. (B) A ribbon diagram of a model for the intramolecular FHA–CC2 interaction and the flexible hinge. The FHA domain (green; aa 488–604) is connected to CC2 (yellow; aa 625–679) via a linker containing a proline-rich region (gray; 13 residues; aa 605–617; prolines at aa 608, 613, and 616) and an α-helix with a weak propensity for coiled-coil formation (wheat; seven residues; aa 618–624). This α-helix merges with the α-helical structure in CC2. The N-terminal region of CC2 contains a small flexible stretch (QGID; aa 630–632) containing a helix-breaking residue (G631). The bidirectional arrow indicates the reversible transition between the bent (yellow) and straight (blue) conformations of CC2. Some side chains are shown by balls and sticks. Our model predicts that the flexible hinge of CC2 allows the intramolecular FHA–CC2 interaction. (C) Increased pull-down of MCFC-G631F. Lysates of HEK293T cells transfected with MCFC-EGFP (wild type, Y649F and G631F) were incubated with GST–CC2 or GST alone, and the precipitates were immunoblotted with EGFP antibodies. Input: 2%. (D, E) Increased MT binding of MCFC-G631F. MT binding of mutant (G631F) MCFC-EGFP was compared to that of MCFC-wild type and MCFC-Y649F (D) and quantified (E). Asterisks in MCFC-G631F (0.60±0.09, _n_=4) and MCFC-Y649F (0.88±0.11, _n_=4) indicate significant changes (*P<0.0001 for both) compared to MCFC-WT (0.14±0.06, _n_=4). (F) Increased peripheral accumulation in MCFC-G631F-expressing neurons. Scale bar: 10 μm.
Similar articles
- Monomeric and dimeric states exhibited by the kinesin-related motor protein KIF1A.
Rashid DJ, Bononi J, Tripet BP, Hodges RS, Pierce DW. Rashid DJ, et al. J Pept Res. 2005 Jun;65(6):538-49. doi: 10.1111/j.1399-3011.2005.00255.x. J Pept Res. 2005. PMID: 15885113 - The CC1-FHA tandem as a central hub for controlling the dimerization and activation of kinesin-3 KIF1A.
Huo L, Yue Y, Ren J, Yu J, Liu J, Yu Y, Ye F, Xu T, Zhang M, Feng W. Huo L, et al. Structure. 2012 Sep 5;20(9):1550-61. doi: 10.1016/j.str.2012.07.002. Epub 2012 Aug 2. Structure. 2012. PMID: 22863567 - Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization.
Tomishige M, Klopfenstein DR, Vale RD. Tomishige M, et al. Science. 2002 Sep 27;297(5590):2263-7. doi: 10.1126/science.1073386. Science. 2002. PMID: 12351789 - Structural links to kinesin directionality and movement.
Wade RH, Kozielski F. Wade RH, et al. Nat Struct Biol. 2000 Jun;7(6):456-60. doi: 10.1038/75850. Nat Struct Biol. 2000. PMID: 10881190 Review. - The UNC-104/KIF1 family of kinesins.
Bloom GS. Bloom GS. Curr Opin Cell Biol. 2001 Feb;13(1):36-40. doi: 10.1016/s0955-0674(00)00171-x. Curr Opin Cell Biol. 2001. PMID: 11163131 Review.
Cited by
- Phosphoregulation of Kinesins Involved in Long-Range Intracellular Transport.
Kumari D, Ray K. Kumari D, et al. Front Cell Dev Biol. 2022 Jun 3;10:873164. doi: 10.3389/fcell.2022.873164. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35721476 Free PMC article. Review. - Structures of kinesin motor proteins.
Marx A, Hoenger A, Mandelkow E. Marx A, et al. Cell Motil Cytoskeleton. 2009 Nov;66(11):958-66. doi: 10.1002/cm.20392. Cell Motil Cytoskeleton. 2009. PMID: 19530174 Free PMC article. Review. - Neurospora crassa NKIN2, a kinesin-3 motor, transports early endosomes and is required for polarized growth.
Seidel C, Moreno-Velásquez SD, Riquelme M, Fischer R. Seidel C, et al. Eukaryot Cell. 2013 Jul;12(7):1020-32. doi: 10.1128/EC.00081-13. Epub 2013 May 17. Eukaryot Cell. 2013. PMID: 23687116 Free PMC article. - The molecular basis for kinesin functional specificity during mitosis.
Welburn JP. Welburn JP. Cytoskeleton (Hoboken). 2013 Sep;70(9):476-93. doi: 10.1002/cm.21135. Epub 2013 Oct 8. Cytoskeleton (Hoboken). 2013. PMID: 24039047 Free PMC article. Review. - The family-specific K-loop influences the microtubule on-rate but not the superprocessivity of kinesin-3 motors.
Soppina V, Verhey KJ. Soppina V, et al. Mol Biol Cell. 2014 Jul 15;25(14):2161-70. doi: 10.1091/mbc.E14-01-0696. Epub 2014 May 21. Mol Biol Cell. 2014. PMID: 24850887 Free PMC article.
References
- Combet C, Blanchet C, Geourjon C, Deleage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25: 147–150 - PubMed
- Cross R, Scholey JM (1999) Kinesin: the tail unfolds. Nat Cell Biol 1: E119–E121 - PubMed
- Dorner C, Ciossek T, Muller S, Moller PH, Ullrich A, Lammers R (1998) Characterization of KIF1C, a new kinesin-like protein involved in vesicle transport from the Golgi apparatus to the endoplasmic reticulum. J Biol Chem 273: 20267–20275 - PubMed
- Durocher D, Jackson SP (2002) The FHA domain. FEBS Lett 513: 58–66 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources