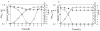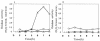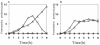Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate - PubMed (original) (raw)
Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate
Masanori Seki et al. J Bacteriol. 2004 Apr.
Abstract
Streptococcus pyogenes strains can be divided into two classes, one capable and the other incapable of producing H2O2 (M. Saito, S. Ohga, M. Endoh, H. Nakayama, Y. Mizunoe, T. Hara, and S. Yoshida, Microbiology 147:2469-2477, 2001). In the present study, this dichotomy was shown to parallel the presence or absence of H2O2-producing lactate oxidase activity in permeabilized cells. Both lactate oxidase activity and H2O2 production under aerobic conditions were detectable only after glucose in the medium was exhausted. Thus, the glucose-repressible lactate oxidase is likely responsible for H2O2 production in S. pyogenes. Of the other two potential H2O2-producing enzymes of this bacterium, NADH and alpha-glycerophosphate oxidase, only the former exhibited low but significant activity in either class of strains. This activity was independent of the growth phase, suggesting that the protein may serve in vivo as a subunit of the H2O2-scavenging enzyme NAD(P)H-linked alkylhydroperoxide reductase. The activity of lactate oxidase was associated with the membrane while that of NADH oxidase was in the soluble fraction, findings consistent with their respective physiological roles, i.e., the production and scavenging of H2O2. Analyses of fermentation end products revealed that the concentration of lactate initially increased with time and decreased on glucose exhaustion, while that of acetate increased during the culture. These results suggest that the lactate oxidase activity of H2O2-producing cells oxidizes lactate to pyruvate, which is in turn converted to acetate. This latter process proceeds presumably via acetyl coenzyme A and acetyl phosphate with formation of extra ATP.
Figures
FIG. 1.
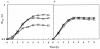
Time course of H2O2 production and glucose consumption in relation to bacterial growth. Strain MK5, an H2O2 producer (A), and strain ME157, an H2O2 nonproducer (B), were cultured in TYG medium at 37°C with shaking. Symbols: ○, turbidity (OD600); ▵, glucose; ×, H2O2; ♦, pH. Representative data from 10 independent experiments are shown.
FIG. 2.
Growth-phase-dependent changes in the activity of H2O2-producing oxidases. Cultures of strain MK5 (A) and strain ME157 (B) were grown in TYG medium at 37°C with shaking, and samples taken at intervals were adjusted to an OD600 of 0.3. The cells in 5-ml portions were collected by centrifugation, resuspended in 0.5 ml of 0.1 M sodium phosphate buffer, and permeabilized by treatment with 10 μl of toluene. The activities were measured with 30 μl of the cell suspension under the conditions described in Materials and Methods and expressed as micromoles of H2O2 formed per minute. Symbols: ○, NADH oxidase; ▵, α-glycerophosphate oxidase; ×, lactate oxidase.
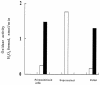
FIG. 3.
Subcellular localization of H2O2-producing oxidases. Cells of strain MK5 grown in TYG medium under the standard conditions for 5 h (see legend to Fig. 1) at 37°C with shaking were harvested and processed as described in Materials and Methods. The supernatant and the pellet denote the soluble and membrane fractions, respectively. The lactate oxidase activity was not detected in the supernatant. The enzyme activity shown represents the total activity contained in each 5-ml preparation (see Materials and Methods). Open bars, NADH oxidase activity; filled bars, lactate oxidase activity.
FIG. 4.
Growth-phase-dependent changes in the concentration of fermentation products in the culture medium. Cells of strain MK5 (A) and strain ME157 (B) were cultured in TYG medium under the standard conditions, and analyses of the products were performed as described in Materials and Methods. Symbols: ○, ethanol; ▵, acetate; ×,
l
-lactate, ♦, formate.
FIG. 5.
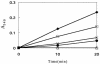
Effect of added lactate on cell yield. The H2O2-producing strain MK5(A) and the nonproducing strain ME157(B) were cultured in TYG medium at 37°C with shaking in the presence of catalase. Stationary-phase cells were harvested, washed, and resuspended in fresh TY medium supplemented with or without 0.1% sodium lactate and catalase (200 U/ml). Symbols: ○, with lactate and catalase; ×, with catalase only; □, with lactate only.
FIG. 6.
Demonstration of NAD+-linked pyruvate cleavage reaction. Permeabilized cells of strain MK5 were used as the enzyme, and the reduction of acetylpyridine NAD+ was monitored by measuring the _A_340. Without the enzyme, this reduction was not observed. Symbols: ♦, complete system; ○, less substrate; •, less thiamine PPi; ×, less coenzyme A; □, less Ca2+; ▵, less Ca2+ but plus 2 mM EDTA.
Similar articles
- Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters.
Quatravaux S, Remize F, Bryckaert E, Colavizza D, Guzzo J. Quatravaux S, et al. J Appl Microbiol. 2006 Oct;101(4):903-12. doi: 10.1111/j.1365-2672.2006.02955.x. J Appl Microbiol. 2006. PMID: 16968302 - Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source.
Taniai H, Iida K, Seki M, Saito M, Shiota S, Nakayama H, Yoshida S. Taniai H, et al. J Bacteriol. 2008 May;190(10):3572-9. doi: 10.1128/JB.01882-07. Epub 2008 Mar 14. J Bacteriol. 2008. PMID: 18344365 Free PMC article. - Hydrogen Peroxide Production of Group A Streptococci (GAS) is emm-Type Dependent and Increased at Low Temperatures.
Menschner L, Falke U, Konrad P, Berner R, Toepfner N. Menschner L, et al. Curr Microbiol. 2019 Jun;76(6):698-705. doi: 10.1007/s00284-019-01683-y. Epub 2019 Apr 6. Curr Microbiol. 2019. PMID: 30955044 - Glucose and lactate metabolism by Actinomyces naeslundii.
Takahashi N, Yamada T. Takahashi N, et al. Crit Rev Oral Biol Med. 1999;10(4):487-503. doi: 10.1177/10454411990100040501. Crit Rev Oral Biol Med. 1999. PMID: 10634585 Review. - A current perspective on hydrogen peroxide production in honey. A review.
Brudzynski K. Brudzynski K. Food Chem. 2020 Dec 1;332:127229. doi: 10.1016/j.foodchem.2020.127229. Epub 2020 Jun 5. Food Chem. 2020. PMID: 32688187 Review.
Cited by
- Bacterial Response to Oxidative Stress and RNA Oxidation.
Seixas AF, Quendera AP, Sousa JP, Silva AFQ, Arraiano CM, Andrade JM. Seixas AF, et al. Front Genet. 2022 Jan 10;12:821535. doi: 10.3389/fgene.2021.821535. eCollection 2021. Front Genet. 2022. PMID: 35082839 Free PMC article. Review. - Evolution of Photorespiratory Glycolate Oxidase among Archaeplastida.
Kern R, Facchinelli F, Delwiche C, Weber APM, Bauwe H, Hagemann M. Kern R, et al. Plants (Basel). 2020 Jan 15;9(1):106. doi: 10.3390/plants9010106. Plants (Basel). 2020. PMID: 31952152 Free PMC article. - SO-LAAO, a novel L-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone.
Tong H, Chen W, Shi W, Qi F, Dong X. Tong H, et al. J Bacteriol. 2008 Jul;190(13):4716-21. doi: 10.1128/JB.00363-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469105 Free PMC article. - Glycine Betaine Monooxygenase, an Unusual Rieske-Type Oxygenase System, Catalyzes the Oxidative _N_-Demethylation of Glycine Betaine in Chromohalobacter salexigens DSM 3043.
Shao YH, Guo LZ, Zhang YQ, Yu H, Zhao BS, Pang HQ, Lu WD. Shao YH, et al. Appl Environ Microbiol. 2018 Jun 18;84(13):e00377-18. doi: 10.1128/AEM.00377-18. Print 2018 Jul 1. Appl Environ Microbiol. 2018. PMID: 29703733 Free PMC article. - H2O2 generation by bacillus Calmette-Guérin induces the cellular oxidative stress response required for bacillus Calmette-Guérin direct effects on urothelial carcinoma biology.
Shah G, Zielonka J, Chen F, Zhang G, Cao Y, Kalyanaraman B, See W. Shah G, et al. J Urol. 2014 Oct;192(4):1238-48. doi: 10.1016/j.juro.2014.05.115. Epub 2014 Jun 10. J Urol. 2014. PMID: 24928267 Free PMC article.
References
- Abbe, K., J. Carlsson, S. Takahashi-Abbe, and T. Yamada. 1991. Oxygen and the sugar metabolism in oral streptococci. Proc. Finn. Dent. Soc. 87:477-487. - PubMed
- Chance, B., H. Sies, and A. Boveris. 1979. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59:527-605. - PubMed
- Futai, M., and H. Kimura. 1977. Inducible membrane-bound l-lactate dehydrogenase from Escherichia coli. Purification and properties. J. Biol. Chem. 252:5820-5827. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources