Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses - PubMed (original) (raw)
Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses
Laura N Antar et al. J Neurosci. 2004.
Abstract
Fragile X syndrome is caused by the absence of the mRNA-binding protein Fragile X mental retardation protein (FMRP), which may play a role in activity-regulated localization and translation of mRNA in dendrites and at synapses. We investigated whether neuronal activity and glutamatergic signals regulate trafficking of FMRP and its encoding Fmr1 mRNA into dendrites or at synapses. Using high-resolution fluorescence and digital imaging microscopy in cultured hippocampal neurons, FMRP and Fmr1 mRNA were localized in granules throughout dendrites and within spines. KCl depolarization rapidly increased FMRP and Fmr1 mRNA levels in dendrites. Metabotropic glutamate receptor (mGluR) activation, in particular mGluR5 activation, was necessary for localization of FMRP into dendrites. Blockade of either PKC or internal calcium prevented mGluR-dependent localization of both FMRP and Fmr1 mRNA in dendrites. The activity-dependent localization of FMRP was not dependent on protein synthesis. Fluorescence recovery after photobleaching analysis of live neurons transfected with enhanced green fluorescent protein-FMRP revealed increased granule trafficking in response to KCl depolarization. In contrast to its dendritic localization, mGluR activation diminished FMRP, but not Fmr1 mRNA, localization at synapses. These results demonstrate regulation of FMRP and Fmr1 mRNA trafficking in dendrites and synapses in response to specific glutamatergic signals.
Figures
Figure 3.
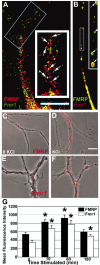
A–E, FRAP analysis of increased EGFP–FMRP granule trafficking in response to KCl stimulation. Hippocampal neurons were transfected with EGFP–FMRP and imaged with either no added stimulus(A, C) or 20 m
m
KCl (B, D). The recovery of each photobleached region was followed for 5 min at 1 min intervals. A, The first panel (from left) shows the EGFP–FMRP signal in an unstimulated neurite before photobleach, and the second panel shows immediately after photobleach. Subsequent panels show signal at 3 min (3rd panel) and 5 min (4th panel) after photobleach. Arrowheads (A, B) point to regions that exemplify the reduced recovery of EGFP–FMRP granules in the absence of stimulation (A) compared with those in the same cell treated with 20 m
m
KCl (B). Quantitative analysis of recovery of the EGFP–FMRP signal in this cell is shown at three time points (15, 30, 60 min) after perfusion with normal media as a control (C) or media with KCl (D) treatment. A summary of all FRAP analyses (E) compares the percentage recovery of EGFP–FMRP in neurites of several cells measured for each treatment within 1 hr of stimulation or mock treatment. F, Dendritic localization of FMRP was not protein synthesis dependent. Bath application of cycloheximide for 30 min to cultured neurons before stimulation (5 min DHPG) did not affect mGluR-dependent FMRP localization.
Figure 1.
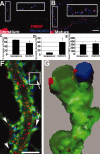
Developmental prevalence of FMRP at synapses. A, IF of an 8 DIV neuron shows frequent FMRP (red) colocalization with synapsin (blue; see enlarged inset). B, Same as A but a mature 19 DIV neuron. C, Histogram showing that 75% of synapsin puncta colocalized with FMRP at 8 DIV; only 54% colocalized at 19 DIV (*p < 0.05; Student's t test). D, Density of synapses increased as neurons matured (p < 0.05). E, There was no difference in FMRP levels in dendritic shafts of 8 and 19 DIV cultures. Scale bar, 10 μm. F, Triple-label IF detects FMRP (red), synapsin (blue), and F-actin (green). FMRP granules are distributed throughout dendrites, spine synapses (arrows), and filopodia (arrowheads). Scale bar, 5μm. G, 3-D reconstruction of boxed spine from F shows FMRP granules in spine neck and head, apposed to synapsin.
Figure 2.
Localization of FMRP and Fmr1 mRNA granules in dendritic compartments and their KCl-induced localization. A, FISH followed by IF shows that FMRP (red) and Fmr1 mRNA (green) localize to dendrites and colocalize (yellow; see arrows in enlarged inset) with each other: 26% FMRP-positive pixels also contain Fmr1 mRNA. Scale bar, 10μm. B, Transfected rat hippocampal neurons show EGFP–FMRP granules colocalized with Fmr1 mRNA (red); see also enlarged inset (arrows). C–G, Time course of KCl-induced localization of FMRP and Fmr1 mRNA in dendrites. C, IF detection of FMRP (red) granules in secondary dendrites from unstimulated neurons (phase). Stimulation with KCl (D, G) caused FMRP IF intensity in secondary dendrites to increase. Increases were sustained for 1 hr and then subsided. E, FISH detection of Fmr1 mRNA granules (red) was apparent in secondary dendrites of unstimulated neurons (phase). After KCl treatment (F, G), Fmr1 mRNA IF intensity in dendrites increased dramatically. Note: fluorescence intensity was thresholded to just above background in unstimulated neurons (C, E) to illustrate the marked increase in intensity on stimulation (C–F). Scale bar, 10 μm.
Figure 4.
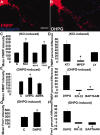
mGluRs regulate FMRP and Fmr1 mRNA localization in dendrites. A, B, E, DHPG stimulation (5 min) resulted in increased FMRP levels in dendrites. C, KCl stimulation (10 min) also increased FMRP levels (p < 0.05) in dendrites. This response was entirely inhibited by an mGluR antagonist, MCPG, but not by the AMPAR antagonist, CNQX. D, Bath application of mGluR5 antagonist MPEP blocked (p < 0.05) the KCl-regulated increase of FMRP in dendrites whereas mGluR1 antagonist LY-367385 did not. Bars indicate percentage difference of control compared with KCl application (KCl), MPEP compared with MPEP plus KCl (MPEP), and LY compared with LY plus KCl (LY). E, Treatment of neurons with the group I mGluR agonist DHPG resulted in increased dendritic FMRP levels (p < 0.05), a response not observed with AMPA. F, Blockade of the mGluR pathway through either PKC inactivation (RO-32) or chelating internal calcium (BAPTA-AM) abolished the dendritic localization of FMRP in response to mGluR activation. G, Fmr1 mRNA also demonstrated mGluR-dependent dendritic trafficking. DHPG activation of mGluR receptors localized the mRNA in dendrites (p < 0.05). H, Blockade of the mGluR pathway, as in F, also prevented Fmr1 mRNA localization. Histograms depict mean FMRP or Fmr1 mRNA IF intensity in response to various stimuli or the percentage difference in immunofluorescence intensity of an antagonist before and after stimulus. Unstimulated cultures in each histogram are labeled “C.” All culture conditions contain antagonists APV and CNQX, except where testing AMPA or NMDA. Scale bar, 10 μm.
Figure 5.
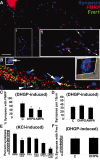
mGluRs regulate FMRP and Fmr1 mRNA localization in synapses. A, Rat (8 DIV) rat hippocampal cultures with double-label IF showing extensive FMRP (red) colocalization with synapsin puncta (blue) along neuronal processes. Boxed inset (1) is rotated and enlarged (at side). Arrows in enlarged inset depict examples of FMRP–synapsin colocalization. B, A combination FISH and double-label IF shows that FMRP and Fmr1 mRNA localize and colocalize in synapses. Concave arrowheads show FMRP localized to synapses; straight arrowheads show Fmr1 mRNA localized to synapses. Full arrows show FMRP and Fmr1 mRNA colocalization within a synapse (see E for quantification). Histogram in C quantified the percentage of synapses with an FMRP punctum associated with them before and after DHPG (5 min) and AMPA (15 min) stimulation (p < 0.05). D, Histogram shows that these short glutamatergic treatments do not affect synaptic density (p < 0.05). E, Histogram depicts changes in percentage synaptic localization of FMRP and Fmr1 mRNA after KCl depolarization in same cells using single-label FISH combined with double-label IF. Bars 1 and 2 show decreased FMRP localization at synapses after KCl stimulation (∼80 to ∼50%). Bars 3 and 4 show that Fmr1 mRNA is not diminished at synapses after KCl stimulation (∼60 to ∼60%). Bars 5 and 6 show percentage synapses with both FMRP and Fmr1 mRNA in them before and after KCl depolarization (∼45 to ∼20%). The loss of FMRP–Fmr1 mRNA colocalization at synapses after stimulation appears to be attributable to the activity-dependent loss of FMRP from synapses. F, mGluR stimulation via DHPG does not affect Fmr1 mRNA localization at dendrites despite the effect of DHPG to diminish FMRP at synapses (C). Scale bar, 10 μm.
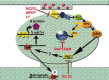
Figure 6.
Model for FMRP and Fmr1 mRNA localization in dendrites and at synapses. Glutamatergic stimulation activates the mGluR, triggering release of internal calcium and activation of PKC, a cascade of events that localizes FMRP and Fmr1 mRNA in dendrites. FMRP and its target mRNA colocalize at the synapse, in a complex where FMRP represses translation. Synaptic activation causes the dissociation of FMRP from the target mRNA, relieving the repression, and the loss of FMRP from the synapse, perhaps because of PKC phosphorylation. Although there is no direct evidence that PKC phosphorylates FMRP, it is known that there is a phosphorylation site on the FMRP protein (Ceman et al., 2003). Phosphorylated FMRP could become a retrograde messenger, returning to the cell body. Meanwhile, the mRNA is now derepressed and able to translate proteins that are important for synaptic structure and plasticity.
Similar articles
- The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain.
Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. Aschrafi A, et al. Proc Natl Acad Sci U S A. 2005 Feb 8;102(6):2180-5. doi: 10.1073/pnas.0409803102. Epub 2005 Jan 31. Proc Natl Acad Sci U S A. 2005. PMID: 15684045 Free PMC article. - Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression.
Niere F, Wilkerson JR, Huber KM. Niere F, et al. J Neurosci. 2012 Apr 25;32(17):5924-36. doi: 10.1523/JNEUROSCI.4650-11.2012. J Neurosci. 2012. PMID: 22539853 Free PMC article. - Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons.
Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Antar LN, et al. Genes Brain Behav. 2005 Aug;4(6):350-9. doi: 10.1111/j.1601-183X.2005.00128.x. Genes Brain Behav. 2005. PMID: 16098134 - Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse.
Ronesi JA, Huber KM. Ronesi JA, et al. Sci Signal. 2008 Feb 5;1(5):pe6. doi: 10.1126/stke.15pe6. Sci Signal. 2008. PMID: 18272470 Review. - Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function.
Bassell GJ, Warren ST. Bassell GJ, et al. Neuron. 2008 Oct 23;60(2):201-14. doi: 10.1016/j.neuron.2008.10.004. Neuron. 2008. PMID: 18957214 Free PMC article. Review.
Cited by
- Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation.
Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Nalavadi VC, et al. J Neurosci. 2012 Feb 22;32(8):2582-7. doi: 10.1523/JNEUROSCI.5057-11.2012. J Neurosci. 2012. PMID: 22357842 Free PMC article. - Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases.
Wang H, Zhuo M. Wang H, et al. Front Pharmacol. 2012 Nov 1;3:189. doi: 10.3389/fphar.2012.00189. eCollection 2012. Front Pharmacol. 2012. PMID: 23125836 Free PMC article. - The unstable repeats--three evolving faces of neurological disease.
Nelson DL, Orr HT, Warren ST. Nelson DL, et al. Neuron. 2013 Mar 6;77(5):825-43. doi: 10.1016/j.neuron.2013.02.022. Neuron. 2013. PMID: 23473314 Free PMC article. Review. - A 'learning platform' approach to outcome measurement in fragile X syndrome: a preliminary psychometric study.
Hall SS, Hammond JL, Hirt M, Reiss AL. Hall SS, et al. J Intellect Disabil Res. 2012 Oct;56(10):947-60. doi: 10.1111/j.1365-2788.2012.01560.x. Epub 2012 Apr 25. J Intellect Disabil Res. 2012. PMID: 22533667 Free PMC article. - Primary Ciliary Deficits in the Dentate Gyrus of Fragile X Syndrome.
Lee B, Panda S, Lee HY. Lee B, et al. Stem Cell Reports. 2020 Aug 11;15(2):454-466. doi: 10.1016/j.stemcr.2020.07.001. Epub 2020 Jul 30. Stem Cell Reports. 2020. PMID: 32735823 Free PMC article.
References
- Aakalu G, Smith WB, Jiang C, Nguyen N, Schuman EM (2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502. - PubMed
- Antar LN, Bassell GJ (2003) Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron 555–558. - PubMed
- Ashley CT, Wilkinson KD, Reines D, Warren ST (1993) FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262: 563–566. - PubMed
- Bonhoeffer T, Yuste R (2002) Spine motility. Phenomenology, mechanisms, and function. Neuron 35: 1019–1027. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases