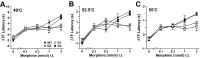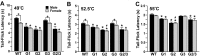Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia - PubMed (original) (raw)
Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia
Cheryl L Marker et al. J Neurosci. 2004.
Abstract
G-protein-gated potassium (K+) channels are found throughout the CNS in which they contribute to the inhibitory effects of neurotransmitters and drugs of abuse. Recent studies have implicated G-protein-gated K+ channels in thermal nociception and the analgesic action of morphine and other agents. Because nociception is subject to complex spinal and supraspinal modulation, however, the relevant locations of G-protein-gated K+ channels are unknown. In this study, we sought to clarify the expression pattern and subunit composition of G-protein-gated K+ channels in the spinal cord and to assess directly their contribution to thermal nociception and morphine analgesia. We detected GIRK1 (G-protein-gated inwardly rectifying K+ channel subunit 1) and GIRK2 subunits, but not GIRK3, in the superficial layers of the dorsal horn. Lack of either GIRK1 or GIRK2 was correlated with significantly lower expression of the other, suggesting that a functional and physical interaction occurs between these two subunits. Consistent with these findings, GIRK1 knock-out and GIRK2 knock-out mice exhibited hyperalgesia in the tail-flick test of thermal nociception. Furthermore, GIRK1 knock-out and GIRK2 knock-out mice displayed decreased analgesic responses after the spinal administration of higher morphine doses, whereas responses to lower morphine doses were preserved. Qualitatively similar data were obtained with wild-type mice after administration of the G-protein-gated K+ channel blocker tertiapin. We conclude that spinal G-protein-gated K+ channels consisting primarily of GIRK1/GIRK2 complexes modulate thermal nociception and mediate a significant component of the analgesia evoked by intrathecal administration of high morphine doses
Figures
Figure 3.
Effect of intrathecal morphine in wild-type and GIRK knock-out mice. Tail-flick latencies were measured at 49°C (A), 52.5°C (B), and 55°C (C), 10 min after the intrathecal injection of saline or morphine (0.1, 0.3, 1, and 3 nmol). The difference between preinjection and postinjection tail-flick latency (ΔTF latency) is plotted as a function of morphine dose. Separate cohorts of mice were used for each drug condition. Wild-type (WT, black circles), GIRK1 knock-out (G1, white circles), GIRK2 knock-out (G2, white squares), and GIRK3 knock-out (G3, black squares) groups were balanced with respect to gender and ranged in size from 11 to 38 animals per dose. Statistical symbols are as follows: *p < 0.05, GIRK1 and GIRK2 knock-out versus wild type (WT) (same dose); +p < 0.05, GIRK2 knock-out versus wild type (same dose).
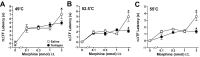
Figure 4.
Effect of intrathecal tertiapin in wild-type mice. Tail-flick latencies of wild-type mice were measured at 49°C (A), 52.5°C (B), and 55°C (C) before and after the intrathecal injection of saline or tertiapin (10, 30, and 100 pmol). The difference between preinjection and postinjection tail-flick latency (ΔTF) was calculated and plotted as a function of tertiapin dose. Separate cohorts of mice were used for each tertiapin dose, and groups (26–29 mice each) were balanced with respect to gender. Tertiapin produced dose-dependent decreases in tail-flick latencies at all three temperatures. *p < 0.05, tertiapin versus saline.
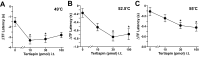
Figure 5.
Tertiapin blunts the analgesic effect of high-dose morphine in wild-type mice. The analgesic effect of morphine (0, 0.1, 0.3, 1, and 3 nmol) coadministered intrathecally with either saline (white circles) or 30 pmol of tertiapin (black circles) was measured in wild-type mice. Preinjection and postinjection tail-flick latencies were measured at 49°C (A), 52.5°C (B), and 55°C (C), and normalized ΔTF latency (nΔTF latency) was calculated as described (see Materials and Methods). Separate cohorts of mice were used for each drug condition. Groups were balanced with respect to gender, and sizes ranged from 11 to 16 mice per drug condition. *p < 0.05, tertiapin versus saline; **p < 0.05, 3 versus 1 nmol of morphine.
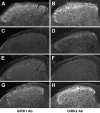
Figure 1.
GIRK subunit expression in the dorsal horn of the spinal cord. Spinal cord sections from wild-type (A, B), GIRK1 knock-out (C, D), GIRK2 knock-out (E, F), and GIRK3 knock-out (G, H) mice were stained with anti-GIRK1 (left column) or anti-GIRK2 (right column) antibodies (Ab). Note the absence of GIRK1 and GIRK2 staining in sections from the corresponding knock-out mice (C, F, respectively) and their dramatic reduction in sections from GIRK2 knock-out (E) and GIRK1 knock-out (D) mice, respectively. Also note the slightly lower levels of GIRK1 in sections from GIRK3 knock-out (G), but not GIRK2 knock-out (H), mice. Results are representative of the data obtained from three complete sets of wild-type and GIRK knock-out mice.
Figure 2.
Baseline tail-flick latencies of wild-type and GIRK knock-out mice. Preinjection tail-flick latencies were measured at 49°C (A), 52.5°C (B), and 55°C (C). Both male (black) and female (gray) mice were tested for each genotype. Group designations and sizes (n = male, female) are as follows: wild-type (WT; n = 83, 85), GIRK1 knock-out (G1; n = 52, 46), GIRK2 knock-out (G2; n = 23, 45), GIRK3knock-out(G3; n = 64, 83), and GIRK2/GIRK3 double knock-out (G2/3; n = 4, 10) mice. Statistical symbols and comparisons are as follows: *p < 0.05, GIRK knock-out versus wild type (same gender); +p < 0.05, male versus female (same genotype); #p < 0.05, GIRK2 knock-out versus GIRK1 knock-out.
Similar articles
- Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3.
Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Jelacic TM, et al. J Biol Chem. 2000 Nov 17;275(46):36211-6. doi: 10.1074/jbc.M007087200. J Biol Chem. 2000. PMID: 10956667 - Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids.
Marker CL, Luján R, Loh HH, Wickman K. Marker CL, et al. J Neurosci. 2005 Apr 6;25(14):3551-9. doi: 10.1523/JNEUROSCI.4899-04.2005. J Neurosci. 2005. PMID: 15814785 Free PMC article. - Hyperalgesia and blunted morphine analgesia in G protein-gated potassium channel subunit knockout mice.
Marker CL, Cintora SC, Roman MI, Stoffel M, Wickman K. Marker CL, et al. Neuroreport. 2002 Dec 20;13(18):2509-13. doi: 10.1097/00001756-200212200-00026. Neuroreport. 2002. PMID: 12499858 - Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys?
Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Ikeda K, et al. Neurosci Res. 2002 Oct;44(2):121-131. doi: 10.1016/s0168-0102(02)00094-9. Neurosci Res. 2002. PMID: 12354627 Review. - Signalling via the G protein-activated K+ channels.
Dascal N. Dascal N. Cell Signal. 1997 Dec;9(8):551-73. doi: 10.1016/s0898-6568(97)00095-8. Cell Signal. 1997. PMID: 9429760 Review.
Cited by
- Biasing G_βγ_ Downstream Signaling with Gallein Inhibits Development of Morphine Tolerance and Potentiates Morphine-Induced Nociception in a Tolerant State.
Sanchez GA, Smrcka AV, Jutkiewicz EM. Sanchez GA, et al. Mol Pharmacol. 2024 Jun 18;106(1):47-55. doi: 10.1124/molpharm.124.000875. Mol Pharmacol. 2024. PMID: 38769020 - Oxycodone: A Current Perspective on Its Pharmacology, Abuse, and Pharmacotherapeutic Developments.
Barrett JE, Shekarabi A, Inan S. Barrett JE, et al. Pharmacol Rev. 2023 Nov;75(6):1062-1118. doi: 10.1124/pharmrev.121.000506. Epub 2023 Jun 15. Pharmacol Rev. 2023. PMID: 37321860 Free PMC article. Review. - βγ G-proteins, but not regulators of G-protein signaling 4, modulate opioid-induced respiratory rate depression.
Danaf J, da Silveira Scarpellini C, Montandon G. Danaf J, et al. Front Physiol. 2023 Apr 6;14:1043581. doi: 10.3389/fphys.2023.1043581. eCollection 2023. Front Physiol. 2023. PMID: 37089428 Free PMC article. - GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders.
Kotajima-Murakami H, Ide S, Ikeda K. Kotajima-Murakami H, et al. Biomedicines. 2022 Oct 13;10(10):2552. doi: 10.3390/biomedicines10102552. Biomedicines. 2022. PMID: 36289814 Free PMC article. Review. - Association of KCNJ6 rs2070995 and methadone response for pain management in advanced cancer at end-of-life.
Ozberk D, Haywood A, Sutherland HG, Yu C, Albury CL, Zunk M, George R, Good P, Griffiths LR, Hardy J, Haupt LM. Ozberk D, et al. Sci Rep. 2022 Oct 19;12(1):17422. doi: 10.1038/s41598-022-21180-w. Sci Rep. 2022. PMID: 36261449 Free PMC article.
References
- Aicher SA, Sharma S, Cheng PY, Liu-Chen LY, Pickel VM (2000) Dual ultrastructural localization of mu-opiate receptors and Substance P in the dorsal horn. Synapse 36: 12–20. - PubMed
- Bettahi I, Marker CL, Roman IM, Wickman K (2002) Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh J Biol Chem 277: 48282–48288. - PubMed
- Blednov Y, Stoffel M, Chang S, Harris R (2001a) Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298: 521–530. - PubMed
- Blednov YA, Stoffel M, Chang SR, Harris RA (2001b) GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav 74: 109–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA011806/DA/NIDA NIH HHS/United States
- R01 MH061933/MH/NIMH NIH HHS/United States
- MH61933/MH/NIMH NIH HHS/United States
- T32 DA07234/DA/NIDA NIH HHS/United States
- P50 DA011806/DA/NIDA NIH HHS/United States
- T32 DA007234/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases