CONSORT statement: extension to cluster randomised trials - PubMed (original) (raw)
CONSORT statement: extension to cluster randomised trials
Marion K Campbell et al. BMJ. 2004.
No abstract available
Figures
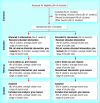
Fig 1
Recommended format for flow diagram of the progress of clusters and individuals through the phases of a randomised trial
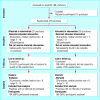
Fig 2
Example of diagram showing flow of clusters and participants through a trial (adapted from Thomas et al38)
Comment in
- Extending CONSORT to include cluster trials.
Campbell MJ. Campbell MJ. BMJ. 2004 Mar 20;328(7441):654-5. doi: 10.1136/bmj.328.7441.654. BMJ. 2004. PMID: 15031214 Free PMC article. No abstract available.
Similar articles
- [The CONSORT statement for cluster randomised trials].
Campbell MK, Elbourne DR, Altman DG; CONSORT CLUSTER group. Campbell MK, et al. Med Clin (Barc). 2005 Dec 1;125 Suppl 1:28-31. doi: 10.1016/s0025-7753(05)72206-5. Med Clin (Barc). 2005. PMID: 16464424 Spanish. - Enhancing CONSORT compliance for improved reporting of randomized controlled trials.
Pandis N, Turpin DL. Pandis N, et al. Am J Orthod Dentofacial Orthop. 2014 Jan;145(1):1. doi: 10.1016/j.ajodo.2013.11.005. Am J Orthod Dentofacial Orthop. 2014. PMID: 24373645 No abstract available. - CONSORT 2010.
[No authors listed] [No authors listed] Lancet. 2010 Apr 3;375(9721):1136. doi: 10.1016/S0140-6736(10)60456-4. Epub 2010 Mar 24. Lancet. 2010. PMID: 20338630 No abstract available. - Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration.
Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, Dixon-Woods M, Aldcroft A, Doussau A, Grayling M, Kristunas C, Goldstein CE, Campbell MK, Girling A, Eldridge S, Campbell MJ, Lilford RJ, Weijer C, Forbes AB, Grimshaw JM. Hemming K, et al. BMJ. 2018 Nov 9;363:k1614. doi: 10.1136/bmj.k1614. BMJ. 2018. PMID: 30413417 Free PMC article. - Consolidated Standards of Reporting Trials (CONSORT) extensions covered most types of randomized controlled trials, but the potential workload for authors was high.
Ghosn L, Boutron I, Ravaud P. Ghosn L, et al. J Clin Epidemiol. 2019 Sep;113:168-175. doi: 10.1016/j.jclinepi.2019.05.030. Epub 2019 May 30. J Clin Epidemiol. 2019. PMID: 31153976 Review.
Cited by
- The effectiveness and cost-effectiveness of attachment-based family therapy for young adults with high suicidal ideation: protocol of a randomized controlled trial.
Bockting C, Bosmans G, Bergers N, Gavan L, Hiligsmann M, de Beurs D, Molenberghs G, Wijnen B, Lokkerbol J, van der Spek N. Bockting C, et al. Trials. 2024 Oct 16;25(1):686. doi: 10.1186/s13063-024-08499-7. Trials. 2024. PMID: 39415182 Free PMC article. - Calculating power for multilevel implementation trials in mental health: Meaningful effect sizes, intraclass correlation coefficients, and proportions of variance explained by covariates.
Williams NJ, Cardamone NC, Beidas RS, Marcus SC. Williams NJ, et al. Implement Res Pract. 2024 Sep 26;5:26334895241279153. doi: 10.1177/26334895241279153. eCollection 2024 Jan-Dec. Implement Res Pract. 2024. PMID: 39346518 Free PMC article. - Pearl millet instant beverage powder enriched with baobab pulp to improve iron and anaemia status of adolescent girls in rural Ghana: a study protocol for a cluster randomised controlled trial.
Atosona A, Larbie C, Apprey C, Annan RA. Atosona A, et al. Br J Nutr. 2024 Sep 14;132(5):565-574. doi: 10.1017/S0007114524001430. Epub 2024 Sep 19. Br J Nutr. 2024. PMID: 39295425 Free PMC article. Clinical Trial. - Cost effectiveness of a GP delivered medication review to reduce polypharmacy and potentially inappropriate prescribing in older patients with multimorbidity in Irish primary care: the SPPiRE cluster randomised controlled trial.
Gillespie P, Moriarty F, Smith SM, Hobbins A, Walsh S, Clyne B, Boland F, McEnteggart T, Flood M, Wallace E, McCarthy C; SPPiRE Study team. Gillespie P, et al. Eur J Health Econ. 2024 Aug 27. doi: 10.1007/s10198-024-01718-7. Online ahead of print. Eur J Health Econ. 2024. PMID: 39190222 - Efficacy and safety of intraperitoneal ropivacaine in pain management following laparoscopic digestive surgery: A systematic review and meta-analysis of RCTs.
Daghmouri MA, Chaouch MA, Deniau B, Benayoun L, Krimi B, Gouader A, Oweira H. Daghmouri MA, et al. Medicine (Baltimore). 2024 Jul 19;103(29):e38856. doi: 10.1097/MD.0000000000038856. Medicine (Baltimore). 2024. PMID: 39029019 Free PMC article.
References
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomised controlled trials. The CONSORT statement. JAMA 1996;276: 637-9. - PubMed
- Moher D, Schulz KF, Altman DG, CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357: 1191-4. - PubMed
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomised trials: explanation and elaboration. Ann Intern Med 2001;134: 663-94. - PubMed
- Fayers PM, Jordhøy MS, Kaasa S. Cluster-randomized trials. Palliat Med 2002;26: 69-70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources