The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins - PubMed (original) (raw)
The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins
Tetyana Klymenko et al. EMBO Rep. 2004 Apr.
Abstract
Transcriptional on and off states of HOX genes and other developmental control genes are maintained by antagonistic regulators encoded by trithorax group (trxG) and Polycomb group (PcG) genes. The trxG proteins Ash1 and hTRX and the PcG repressor E(z) are histone methyltransferases (HMTases) that methylate distinct lysine residues in the N-terminal tail of histone H3. trxG proteins are generally thought to function as activators of HOX genes, but how histone methylation by Ash1 and Trx promotes HOX gene transcription is not clear. Here, we show that in ash1 and trx mutants expression of HOX genes is lost within their normal expression domains, but we find that, contrary to expectation, this expression is restored in ash1 and trx mutants that also lack PcG gene function. Moreover, such trxG PcG double mutants show severe misexpression of HOX genes and, hence, ectopic activation of HOX genes caused by the removal of PcG gene function also occurs in the absence of ash1 and trx function. Together, these results suggest that the Ash1 and Trx HMTases are not "coactivators" required for transcriptional activation of HOX genes, but function specifically as anti-repressors. We propose that histone methylation by Ash1 and Trx is required continuously throughout development to prevent inappropriate PcG silencing of HOX genes in cells in which they must stay transcriptionally active.
Figures
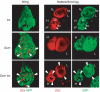
Figure 1
HOX gene expression in trx and Scm single-mutant clones and in Scm trx double-mutant clones. Wing, haltere (H) and third leg (L) imaginal discs stained with antibodies against GFP (green) and Ubx protein (red); clones of mutant cells (negative for GFP) were induced 96 h before analysis. In wild-type animals, Ubx is expressed in haltere and third leg discs but not in the wing disc. Top: Most trx single-mutant clones in haltere and third leg discs show complete loss of Ubx signal (empty arrowhead); in rare cases, some trx mutant cells within a clone maintain Ubx expression at wild-type levels (small arrow). Middle: Scm mutant clones in wing discs show strong misexpression of Ubx (filled arrowheads); clones in haltere and third leg discs show wild-type expression levels. Bottom: Scm trx double-mutant clones in wing discs show strong misexpression at levels comparable to Scm single-mutant clones. Note that Scm trx double-mutant clones in haltere and third leg discs show wild-type levels of Ubx signal (compare with trx single-mutant clones).
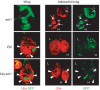
Figure 2
HOX gene expression in ash1 and E(z) single-mutant clones and in E(z) ash1 double-mutant clones. Wing, haltere (H) and third leg (L) imaginal discs stained with antibodies against GFP (green) and Ubx protein (red); clones of mutant cells (negative for GFP) were induced 96 h before analysis. The Minute technique was used in this experiment, resulting in larger clones. Top: ash1 single-mutant clones in haltere and third leg discs show complete loss of Ubx signal (empty arrowhead), but a significant fraction of mutant cells shows undiminished Ubx expression (small arrows). Middle: E(z) mutant clones in the wing disc show strong misexpression of Ubx (filled arrowheads); clones in the haltere and third leg disc show wild-type expression levels. Bottom: E(z) ash1 double-mutant clones in wing discs show strong misexpression at levels comparable to E(z) single-mutant clones. Note that E(z) ash1 double-mutant clones in haltere and third leg discs all show wild-type levels of Ubx signal (compare with ash1 single-mutant clones).
Figure 3
HOX genes are misexpressed in PcG trxG double-mutant embryos. Ventral views of embryonic cuticles (A, left column) and stage-16 embryos stained with antibody against Abd-B protein (A (right column), B). In cuticle images, the white bar marks the boundary between thoracic and abdominal segments, and the arrow in wt marks the eighth abdominal segment. In stained embryos, the anterior margin of parasegment 10 is marked by a black bar. Genotypes are as indicated; ‘m–z–' lack maternal and zygotic product, ‘z–' lack zygotic product. (A) In wt embryos, denticle belts in each segment have a characteristic shape (left) and Abd-B is expressed from ps 10 to ps 14 (right). Cuticles of trx m–z– and trx z– embryos are indistinguishable and show only mild homeotic transformations (for details, see Ingham, 1983); Abd-B expression is reduced in the CNS (empty arrowhead) and epidermis (not visible in this focal plane). Cuticles of Scm m–z–, Scm m–z– trx m–z– and esc m–z– embryos are indistinguishable; they show complete homeotic transformation of all segments into copies of the eighth abdominal segment and strong misexpression of Abd-B in all segments. esc m–z– trx z– embryos show partial suppression of homeotic transformations in the cuticle (i.e. thoracic denticle belts are comparable to those in wt embryos), but the head is abnormal and abdominal segments are still partly transformed towards the eighth abdominal segment; Abd-B is misexpressed in all segments, but expression levels are reduced compared with esc m–z embryos. For unknown reasons, Abd-B expression in ps 13 and 14 of esc m–z– trx z– mutant embryos is lower than in wt, trx z– or esc m–z single-mutant embryos. (B) ash1 m–z embryos show reduction of Abd-B expression similar to trx z– mutant embryos (empty arrowheads). ash1 z– trx z– mutant embryos show slightly more extensive reduction of Abd-B expression (empty arrowheads). ash1 z– Scm z– trx z– triple-mutant embryos show severe misexpression of Abd-B comparable to Scm z– or Scm z– trx z– mutant embryos, but note that expression levels in the epidermis are slightly reduced.
Similar articles
- Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins.
Papp B, Müller J. Papp B, et al. Genes Dev. 2006 Aug 1;20(15):2041-54. doi: 10.1101/gad.388706. Genes Dev. 2006. PMID: 16882982 Free PMC article. - The trithorax group proteins Kismet and ASH1 promote H3K36 dimethylation to counteract Polycomb group repression in Drosophila.
Dorighi KM, Tamkun JW. Dorighi KM, et al. Development. 2013 Oct;140(20):4182-92. doi: 10.1242/dev.095786. Epub 2013 Sep 4. Development. 2013. PMID: 24004944 Free PMC article. - Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA polymerase II.
Srinivasan S, Dorighi KM, Tamkun JW. Srinivasan S, et al. PLoS Genet. 2008 Oct;4(10):e1000217. doi: 10.1371/journal.pgen.1000217. Epub 2008 Oct 10. PLoS Genet. 2008. PMID: 18846226 Free PMC article. - The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3.
Cao R, Zhang Y. Cao R, et al. Curr Opin Genet Dev. 2004 Apr;14(2):155-64. doi: 10.1016/j.gde.2004.02.001. Curr Opin Genet Dev. 2004. PMID: 15196462 Review. - Polycomb and Trithorax Group Genes in Drosophila.
Kassis JA, Kennison JA, Tamkun JW. Kassis JA, et al. Genetics. 2017 Aug;206(4):1699-1725. doi: 10.1534/genetics.115.185116. Genetics. 2017. PMID: 28778878 Free PMC article. Review.
Cited by
- Recognition of methylated peptides by Drosophila melanogaster polycomb chromodomain.
Stein RS, Li N, He W, Komives E, Wang W. Stein RS, et al. J Proteome Res. 2013 Mar 1;12(3):1467-77. doi: 10.1021/pr3011205. Epub 2013 Feb 4. J Proteome Res. 2013. PMID: 23320494 Free PMC article. - Genetic Dissection Reveals the Role of Ash1 Domains in Counteracting Polycomb Repression.
Dorafshan E, Kahn TG, Glotov A, Savitsky M, Schwartz YB. Dorafshan E, et al. G3 (Bethesda). 2019 Nov 5;9(11):3801-3812. doi: 10.1534/g3.119.400579. G3 (Bethesda). 2019. PMID: 31540973 Free PMC article. - Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains.
Jiang H, Shukla A, Wang X, Chen WY, Bernstein BE, Roeder RG. Jiang H, et al. Cell. 2011 Feb 18;144(4):513-25. doi: 10.1016/j.cell.2011.01.020. Cell. 2011. PMID: 21335234 Free PMC article. - Roles and regulation of histone methylation in animal development.
Jambhekar A, Dhall A, Shi Y. Jambhekar A, et al. Nat Rev Mol Cell Biol. 2019 Oct;20(10):625-641. doi: 10.1038/s41580-019-0151-1. Epub 2019 Jul 2. Nat Rev Mol Cell Biol. 2019. PMID: 31267065 Free PMC article. Review. - Silencing chromatin: comparing modes and mechanisms.
Beisel C, Paro R. Beisel C, et al. Nat Rev Genet. 2011 Feb;12(2):123-35. doi: 10.1038/nrg2932. Epub 2011 Jan 11. Nat Rev Genet. 2011. PMID: 21221116 Review.
References
- Beisel C, Imhof A, Greene J, Kremmer E, Sauer F (2002) Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419: 857–862 - PubMed
- Beuchle D, Struhl G, Müller J (2001) Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development 128: 993–1004 - PubMed
- Breen TR, Harte PJ (1993) Trithorax regulates multiple homeotic genes in the bithorax and Antennapedia complexes and exerts different tissuespecific, parasegment-specific and promoter-specific effects on each. Development 117: 119–134 - PubMed
- Cao R et al. (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases