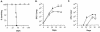MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression - PubMed (original) (raw)
MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression
Charles A Scanga et al. Infect Immun. 2004 Apr.
Abstract
Mycobacterium tuberculosis possesses agonists for several Toll-like receptors (TLRs), yet mice with single TLR deletions are resistant to acute tuberculosis. MyD88(-/-) mice were used to examine whether TLRs play any role in protection against aerogenic M. tuberculosis H37Rv infection. MyD88(-/-) mice failed to control mycobacterial replication and rapidly succumbed. Moreover, expressions of interleukin 12, tumor necrosis factor alpha, gamma interferon, and nitric oxide synthase 2 were markedly decreased in the knockout animals. These results argue that resistance to M. tuberculosis must depend on MyD88-dependent signals mediated by an as-yet-undetermined TLR or a combination of TLRs.
Figures
FIG. 1.
MyD88−/− mice are more susceptible than WT mice to aerogenic M. tuberculosis infection. (A) MyD88−/− mice (diamonds) and C57BL/6 × 129sv (F1) WT animals (squares), used to control for the possible influence of contaminating 129 genes, were infected in groups of 5 to 7 mice with 20 to 50 CFU of M. tuberculosis and monitored for survival. Data are representative of two separate experiments. Bacterial burdens (means ± standard errors) in the lungs (B) or livers (C) were also measured in three mice per group at the times indicated. Asterisks indicate statistically significant (P ≤ 0.05) differences determined by unpaired t test after log transformation between CFU values in MyD88−/− and WT animals. Data are representative of the results from two experiments.
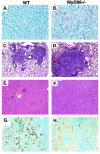
FIG. 2.
MyD88−/− mice infected with M. tuberculosis exhibit more bacilli, exacerbated pathology, and reduced NOS2 expression in lungs, as well as delayed granuloma formation in liver. Formalin-fixed, paraffin-embedded lung (A to D, G, and H) and liver (E and F) tissue sections from mice 3 weeks after infection with aerosol M. tuberculosis were stained by the Kinyoun acid-fast method to detect mycobacteria (red staining) (A and B) or with hematoxylin and eosin stain (C to F). Note the absence of granulomas in panel F. NOS2 was visualized immunohistochemically in serial sections of these same tissues (G and H). Sections shown are representative of multiple fields from the organs of at least three animals per group. Original magnifications are ×63 (A and B), ×5 (C and D), ×10 (E and F), and ×20 (G and H).
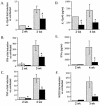
FIG. 3.
Expression of IL-12, IFN-γ, and TNF-α, as well as NOS2, is impaired in MyD88−/− mice following aerogenic M. tuberculosis infection. Real-time RT-PCR was used to quantitate IL-12p40 (A), IFN-γ (B), TNF-α (C), and NOS2 (F) mRNA expression in the lungs of infected WT (gray bars) and MyD88−/− (black bars) mice. In parallel experiments, splenocytes from infected WT and MyD88−/− mice were isolated and restimulated with purified protein derivative, and the supernatants were analyzed 3 days later for IL-12p40 (D) and IFN-γ (E) by enzyme-linked immunosorbent assay. Each bar is the mean (± standard deviation) of data from three mice. Asterisks indicate a P value of ≤0.05 determined by unpaired t test. Data are representative of results from three experiments.
Similar articles
- Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88.
Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fremond CM, et al. J Clin Invest. 2004 Dec;114(12):1790-9. doi: 10.1172/JCI21027. J Clin Invest. 2004. PMID: 15599404 Free PMC article. - Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9.
Hölscher C, Reiling N, Schaible UE, Hölscher A, Bathmann C, Korbel D, Lenz I, Sonntag T, Kröger S, Akira S, Mossmann H, Kirschning CJ, Wagner H, Freudenberg M, Ehlers S. Hölscher C, et al. Eur J Immunol. 2008 Mar;38(3):680-94. doi: 10.1002/eji.200736458. Eur J Immunol. 2008. PMID: 18266299 - A universal role for MyD88 in TLR/IL-1R-mediated signaling.
Janssens S, Beyaert R. Janssens S, et al. Trends Biochem Sci. 2002 Sep;27(9):474-82. doi: 10.1016/s0968-0004(02)02145-x. Trends Biochem Sci. 2002. PMID: 12217523 Review. - MyDths and un-TOLLed truths: sensor, instructive and effector immunity to tuberculosis.
Reiling N, Ehlers S, Hölscher C. Reiling N, et al. Immunol Lett. 2008 Feb 15;116(1):15-23. doi: 10.1016/j.imlet.2007.11.015. Epub 2007 Dec 26. Immunol Lett. 2008. PMID: 18191460 Review.
Cited by
- Granulomas and Inflammation: Host-Directed Therapies for Tuberculosis.
Ndlovu H, Marakalala MJ. Ndlovu H, et al. Front Immunol. 2016 Oct 24;7:434. doi: 10.3389/fimmu.2016.00434. eCollection 2016. Front Immunol. 2016. PMID: 27822210 Free PMC article. Review. - CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis.
Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der Poll T. Wieland CW, et al. Immunology. 2008 Oct;125(2):272-9. doi: 10.1111/j.1365-2567.2008.02840.x. Epub 2008 Apr 3. Immunology. 2008. PMID: 18393969 Free PMC article. - Mycobacterium tuberculosis Hip1 dampens macrophage proinflammatory responses by limiting toll-like receptor 2 activation.
Madan-Lala R, Peixoto KV, Re F, Rengarajan J. Madan-Lala R, et al. Infect Immun. 2011 Dec;79(12):4828-38. doi: 10.1128/IAI.05574-11. Epub 2011 Sep 26. Infect Immun. 2011. PMID: 21947769 Free PMC article. - Alterations in ubiquitin ligase Siah-2 and its corepressor N-CoR after P-MAPA immunotherapy and anti-androgen therapy: new therapeutic opportunities for non-muscle invasive bladder cancer.
Garcia PV, Apolinário LM, Böckelmann PK, da Silva Nunes I, Duran N, Fávaro WJ. Garcia PV, et al. Int J Clin Exp Pathol. 2015 May 1;8(5):4427-43. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191134 Free PMC article. - TB, or not TB: that is the question -- does TLR signaling hold the answer?
Doherty TM, Arditi M. Doherty TM, et al. J Clin Invest. 2004 Dec;114(12):1699-703. doi: 10.1172/JCI23867. J Clin Invest. 2004. PMID: 15599394 Free PMC article. Review.
References
- Abel, B., N. Thieblemont, V. J. F. Quesniaux, N. Brown, J. Mpagi, K. Miyake, F. Bihl, and B. Ryffel. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155-3162. - PubMed
- Adachi, K., H. Tsutsui, S.-I. Kashiwamura, E. Seki, H. Nakano, O. Takeuchi, K. Takeda, K. Okumura, L. Van Kaer, H. Okamura, S. Akira, and K. Nakanishi. 2001. Plasmodium berghei infection in mice induces liver injury by an IL-12 and Toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 167:5928-5934. - PubMed
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
- Brightbill, H. D., D. H. Libratey, S. R. Krutzik, R. B. Yang, J. T. Belisle, S. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. - PubMed
- Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases