Identifying epitopes of HIV-1 that induce protective antibodies - PubMed (original) (raw)
Review
Identifying epitopes of HIV-1 that induce protective antibodies
Susan Zolla-Pazner. Nat Rev Immunol. 2004 Mar.
Abstract
During the past 20 years, the pendulum of opinion in the HIV-1 vaccine field has swung between two extremes, initially favouring the induction of antibodies only, and subsequently favouring the induction of cell-mediated immune responses only. At present, the consensus seems to be that induction of both humoral and cellular immunity by an HIV-1 vaccine will be required to achieve maximum protection. One obstacle to the development of an effective HIV-1 vaccine has been the difficulty in inducing broadly reactive, potent antibodies with protective functions. Defining epitopes and designing immunogens that will induce these antibodies is one of the main challenges that now confronts the HIV-1 vaccine field.
Conflict of interest statement
The author declares no competing financial interests.
Figures
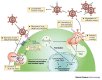
Figure 1. Steps at which antibodies can potentially interfere with virus replication, using HIV-1 as an example.
a | Antibodies can block the virus–target-cell interaction by several mechanisms, such as by: inhibiting the interaction of cell-derived molecules (such as adhesion molecules and lectins) carried in the virus envelope with their ligands on the surface of target cells; inhibiting the binding of virions to CD4 and co-receptors on the cell surface; and preventing conformational changes of the virus envelope that are required for subsequent steps in the virus life cycle. b | After attachment of the virus to target cells, antibodies can inhibit further conformational changes in the virus envelope glycoproteins that create or expose domains involved in virus–target-cell fusion. c | Antibodies can also block the protein domains that are involved in virus–cell fusion. d | At later stages in the virus life cycle, antibodies might be involved in: preventing virus uncoating after entry; interrupting virus assembly; preventing maturation of the virus particle; and inhibiting virus budding. e | Additional mechanisms of antibody-mediated neutralization include complement-induced virolysis and aggregation of infectious virions.
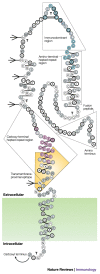
Figure 2. Diagram of the structure of the HIV-1 envelope glycoprotein gp120.
The gp120 molecule with the location of the variable regions marked in boxes (V1–V5). The glycosylation sites containing high mannose-type and/or hybrid-type oligosaccharide structures are indicated by the branched structures, and glycosylation sites containing complex-type oligosaccharide structures are indicated by the U-shaped branches. Epitopes in gp120 that induce neutralizing antibodies are highlighted in colour. These include the highly conformational CD4-binding domain (key epitopes highlighted in yellow), the CD4-induced epitope (green), an epitope composed of α1→2 mannose residues (purple), the V2 loop (orange) and the V3 loop (blue). Reproduced with permission from Ref. © (1990) American Society for Biochemistry and Molecular Biology.
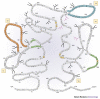
Figure 3. Diagram of the structure of the HIV-1 envelope glycoprotein gp41.
The gp41 molecule with α-helices depicted as alternating three- and four-amino-acid groupings connected by single lines, hydrophobic amino acids indicated as grey circles, charged amino acids as unfilled circles and neutral amino acids as heavily outlined circles. Strong turns are indicated by 'T', and potential glycosylation sites by branched-stick figures. Key functional and structural regions are boxed, and well-characterized epitopes are shown in colour, with epitope clusters I and II shown in turquoise and pink, respectively, and the transmembrane-proximal epitope highlighted in yellow. Reproduced with permission from Ref. © (1989) Mary Ann Liebert Inc. Publishers.
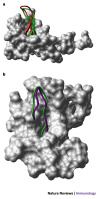
Figure 4. Alternative conformations of HIV-1 V3 loops mimic β-hairpin structures of chemokines.
a | A space-filled diagram of CXC-chemokine ligand 12 (CXCL12, also known as stromal-cell-derived factor 1, SDF1) with the β-hairpin structure shown as a ribbon diagram. The structure of the V3 loop of gp120 (as defined by nuclear magnetic resonance (NMR) analysis when complexed with mouse V3-specific monoclonal antibody 0.5β) is shown in red, superimposed on the β-hairpin of CXCL12, shown in green. b | A space-filled diagram of CC-chemokine ligand 5 (CCL5, also known as RANTES) with the β-hairpin structure shown as a ribbon diagram. The structure of the V3 loop (as defined by NMR analysis when complexed with human V3-specific monoclonal antibody 447/52-D) is shown in purple, superimposed on the β-hairpin of CCL5, shown in green. Images courtesy of M. Schapira.
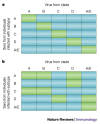
Figure 5. Hypothetical neutralization matrices.
Positive neutralization by a serum–virus pair is denoted by a green box, and the absence of neutralization is denoted by a blue box. If antibodies preferentially neutralized the virus subtype infecting the patient, then neutralization by sera from subjects infected with subtypes A, B, C, D or A/E would preferentially neutralize viruses from the same subtype, as shown in part a. In fact, patterns approximating part b have been achieved in several studies,,, indicating that there is little correlation between the neutralizing activity of a patient's serum and the genotype of the virus infecting that patient.
Similar articles
- HIV vaccine design and the neutralizing antibody problem.
Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. Burton DR, et al. Nat Immunol. 2004 Mar;5(3):233-6. doi: 10.1038/ni0304-233. Nat Immunol. 2004. PMID: 14985706 No abstract available. - HIV vaccines. Magic of the occult?
Montefiori D, Moore JP. Montefiori D, et al. Science. 1999 Jan 15;283(5400):336-7. doi: 10.1126/science.283.5400.336. Science. 1999. PMID: 9925493 No abstract available. - Mapping the immunogenic landscape of near-native HIV-1 envelope trimers in non-human primates.
Cottrell CA, van Schooten J, Bowman CA, Yuan M, Oyen D, Shin M, Morpurgo R, van der Woude P, van Breemen M, Torres JL, Patel R, Gross J, Sewall LM, Copps J, Ozorowski G, Nogal B, Sok D, Rakasz EG, Labranche C, Vigdorovich V, Christley S, Carnathan DG, Sather DN, Montefiori D, Silvestri G, Burton DR, Moore JP, Wilson IA, Sanders RW, Ward AB, van Gils MJ. Cottrell CA, et al. PLoS Pathog. 2020 Aug 31;16(8):e1008753. doi: 10.1371/journal.ppat.1008753. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866207 Free PMC article. - Epitopes of HIV-1 glycoproteins recognized by the human immune system.
Laal S, Zolla-Pazner S. Laal S, et al. Chem Immunol. 1993;56:91-111. Chem Immunol. 1993. PMID: 7680869 Review. No abstract available. - B cell antigenic site mapping of HIV-1 glycoproteins.
Neurath AR. Neurath AR. Chem Immunol. 1993;56:34-60. Chem Immunol. 1993. PMID: 8452653 Review. No abstract available.
Cited by
- Mumps Outbreaks in Vaccinated Populations-Is It Time to Re-assess the Clinical Efficacy of Vaccines?
Connell AR, Connell J, Leahy TR, Hassan J. Connell AR, et al. Front Immunol. 2020 Sep 18;11:2089. doi: 10.3389/fimmu.2020.02089. eCollection 2020. Front Immunol. 2020. PMID: 33072071 Free PMC article. Review. - PATIENT RECRUITMENT USING ELECTRONIC HEALTH RECORDS UNDER SELECTION BIAS: A TWO-PHASE SAMPLING FRAMEWORK.
Zhang G, Beesley LJ, Mukherjee B, Shi XU. Zhang G, et al. Ann Appl Stat. 2024 Sep;18(3):1858-1878. doi: 10.1214/23-aoas1860. Epub 2024 Aug 5. Ann Appl Stat. 2024. PMID: 39149424 Free PMC article. - Evaluation of CD4-CD4i antibody architectures yields potent, broadly cross-reactive anti-human immunodeficiency virus reagents.
West AP Jr, Galimidi RP, Foglesong CP, Gnanapragasam PN, Klein JS, Bjorkman PJ. West AP Jr, et al. J Virol. 2010 Jan;84(1):261-9. doi: 10.1128/JVI.01528-09. J Virol. 2010. PMID: 19864392 Free PMC article. - Soluble CD4 broadens neutralization of V3-directed monoclonal antibodies and guinea pig vaccine sera against HIV-1 subtype B and C reference viruses.
Wu X, Sambor A, Nason MC, Yang ZY, Wu L, Zolla-Pazner S, Nabel GJ, Mascola JR. Wu X, et al. Virology. 2008 Oct 25;380(2):285-95. doi: 10.1016/j.virol.2008.07.007. Epub 2008 Sep 18. Virology. 2008. PMID: 18804254 Free PMC article.
References
- Nishimura Y, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. - DOI - PMC - PubMed
- Letvin NL, et al. Vaccine-elicited V3 loop-specific antibodies in rhesus monkeys and control of a simian-human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate envelope. J. Virol. 2001;75:4165–4175. doi: 10.1128/JVI.75.9.4165-4175.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous