Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity - PubMed (original) (raw)
Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity
Richard J Windle et al. J Neurosci. 2004.
Abstract
We reported previously that the neuropeptide oxytocin attenuates stress-induced hypothalamo-pituitary-adrenal (HPA) activity and anxiety behavior. This study sought to identify forebrain target sites through which oxytocin may mediate its anti-stress effects. Ovariectomized, estradiol-treated rats received intracerebroventricular infusions of oxytocin (1 or 10 ng/hr) or vasopressin (10 ng/hr), and the patterns of neuronal activation after restraint stress were determined by semiquantitative mapping of c-fos mRNA expression. Oxytocin administration significantly attenuated the release of ACTH and corticosterone and the increase in corticotropin-releasing factor mRNA expression in the hypothalamic paraventricular nucleus (PVN) in response to 30 min restraint. Restraint also induced the expression of c-fos mRNA in selective regions of the forebrain, including the PVN, paraventricular thalamic nucleus, habenula, medial amygdala, ventrolateral septum (LSV), most subfields of the dorsal and ventral hippocampus, and piriform and endopiriform cortices. In most cases, this level of gene expression was unaffected by concomitant administration of oxytocin. However, in the PVN, LSV, and throughout all subfields of the dorsal hippocampus, restraint evoked no detectable increase in c-fos mRNA in animals treated with either dose of oxytocin. Vasopressin had no effects on either HPA axis responses or neuronal activation in response to restraint, indicating that the effects were highly peptide selective. These data show that central oxytocin attenuates both the stress-induced neuroendocrine and molecular responses of the HPA axis and that the dorsal hippocampus, LSV, and PVN constitute an oxytocin-sensitive forebrain stress circuit.
Figures
Figure 1.
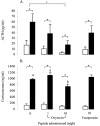
The effect of central oxytocin and vasopressin administration on plasma ACTH (A) and corticosterone (B) concentrations in control animals (open bars) or after a 30 min period of restraint (filled bars). Groups of ovariectomized, estradiol-treated rats were infused centrally with either saline or saline containing 1 or 10 ng/hr oxytocin or 10 ng/hr vasopressin for a period of 5 d before restraint. Values represent the mean ±SE; n = 8–12.*p < 0.05, one-tailed t test versus nonrestrained controls infused with the same treatment; +p < 0.05, post hoc Tukey's test in ANOVA; a, b, bars labeled with different letters differ significantly by post hoc Tukey's test in which a significant interaction was detected in the ANOVA.
Figure 2.
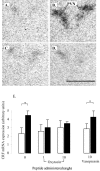
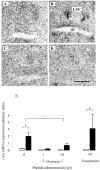
The effect of central infusion of oxytocin and vasopressin on restraint-induced CRF mRNA expression in the PVN. A–D, Images of autoradiograms demonstrating the distribution of hybridized CRF mRNA in the PVN of nonrestrained (A, C) and restrained (B, D) ovariectomized, estradiol-treated rats infused centrally with either saline (A, B) or 10 ng/hr oxytocin (C, D). Scale bar, 100 μm. Note that the marked expression of CRF mRNA induced by restraint in the saline-infused animal (B) is absent in the oxytocin-infused animal (D). E, CRF mRNA expression levels in control animals (open bars) or after a 30 min period of restraint (filled bars). Values shown are the mean ± SE of integrated optical density measurements in arbitrary units (n = 4–6). *p < 0.05, one-tailed t test versus nonrestrained controls infused with the same treatment.
Figure 3.
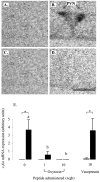
Effect of central infusion of oxytocin and vasopressin on restraint-induced c-fos mRNA expression in the PVN. A–D, Images of representative autoradiograms showing the hybridized c-fos mRNA signal in the PVN of nonrestrained (A, C) and restrained (B, D) ovariectomized, estradiol-treated rats infused centrally with either saline (A, B) or 10 ng/hr oxytocin (C, D). The restraint-induced signal in the saline-infused animal (B) is absent in the oxytocin-infused animal (D). Scale bar, 100 μm. Note that, in all cases, the presence of the PVN was confirmed in adjacent sections. E, Values for mean ± SE (n = 6–8) integrated optical density measurements of c-fos mRNA expression in the PVN of control animals (open bars) and animals after a 30 min restraint (filled bars). *p < 0.05, one-tailed t test versus nonrestrained controls infused with the same treatment; a, b, bars labeled with different letters differ significantly by post hoc Tukey's test in which a significant interaction was detected in the ANOVA.
Figure 4.
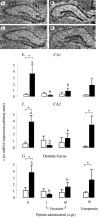
Effect of oxytocin and vasopressin on restraint-induced c-fos mRNA expression in dorsal hippocampus. A–D, Images of representative autoradiograms showing the distribution ofhybridizedc-_fos_mRNA in the dorsal hippocampus of nonrestrained (A,C) and restrained (B,D) ovariectomized, estradiol-treated rats infused centrally with saline (A, B) or 10 ng/hr oxytocin (C, D). The subfields indicated are CA1, CA2, and dentate gyrus (DG). Scale bar, 100 μm. c-fos mRNA expression is lower in restrained animals treated with oxytocin (D) than those infused with saline (B). E, F, Values of mean ± SE (n = 5–8) integrated optical density measurements of c-fos mRNA expression in the CA1 (E), CA2 (F), and dentate gyrus (G) of control animals (open bars) and animals after a 30 min restraint (filled bars). *p < 0.05, one-tailed t test versus nonrestrained controls infused with the same treatment; a, b, bars labeled with different letters differ significantly by post hoc Tukey's test in which a significant interaction was detected in the ANOVA.
Figure 5.
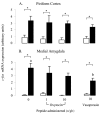
Effect of oxytocin and vasopressin on restraint-induced c-fos mRNA expression in the LSV. A–D, Images of representative autoradiograms showing the distribution of hybridized c-fos mRNA in the LSV of nonrestrained (A, C) and restrained (B, D) ovariectomized, estradiol-treated rats infused centrally with saline (A, B) or 10 ng/hr oxytocin (C, D). Note the clear area of hybridized signal just above the anterior commissure (ac) of the saline-infused restrained animal. Scale bar, 100 μm. E, Mean ± SE (n = 6–8) integrated optical density measurements of c-fos mRNA expression in the LSV of control animals (open bars) or animals undergoing a 30 min period of restraint (filled bars). *p < 0.05, compared with nonrestrained controls infused with the same treatment. +p < 0.05, post hoc Tukey's test in ANOVA.
Figure 6.
The effect of central oxytocin and vasopressin administration on c-fos mRNA expression within the piriform cortex (A) and medial amygdala (B) of control animals (open bars) or after a 30 min period of restraint (filled bars). Groups of ovariectomized, estradiol-treated rats were infused with saline or saline containing either oxytocin (1 or 10 ng/hr) or vasopressin (10 ng/hr) for a period of 5 d before study. Values are the mean ± SE of integrated optical density measurements (n = 6–8). *p < 0.05, one-tailed t test versus nonrestrained controls infused with the same treatment; a, b, bars labeled with different letters differ significantly by post hoc Tukey's test in which a significant interaction was detected in the ANOVA.
Similar articles
- Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat.
Larsen PJ, Tang-Christensen M, Jessop DS. Larsen PJ, et al. Endocrinology. 1997 Oct;138(10):4445-55. doi: 10.1210/endo.138.10.5270. Endocrinology. 1997. PMID: 9322962 - "Green odor" inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions.
Ito A, Miyoshi M, Ueki S, Fukada M, Komaki R, Watanabe T. Ito A, et al. Neurosci Res. 2009 Oct;65(2):166-74. doi: 10.1016/j.neures.2009.06.012. Epub 2009 Jun 27. Neurosci Res. 2009. PMID: 19563846 - Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis.
Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Figueiredo HF, et al. Endocrinology. 2003 Dec;144(12):5249-58. doi: 10.1210/en.2003-0713. Epub 2003 Aug 21. Endocrinology. 2003. PMID: 12960031 - A physiological role for adrenomedullin in rats; a potent hypotensive peptide in the hypothalamo-neurohypophysial system.
Ueta Y, Serino R, Shibuya I, Kitamura K, Kangawa K, Russell JA, Yamashita H. Ueta Y, et al. Exp Physiol. 2000 Mar;85 Spec No:163S-169S. doi: 10.1111/j.1469-445x.2000.tb00020.x. Exp Physiol. 2000. PMID: 10795919 Review. - Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress.
Jankord R, Herman JP. Jankord R, et al. Ann N Y Acad Sci. 2008 Dec;1148:64-73. doi: 10.1196/annals.1410.012. Ann N Y Acad Sci. 2008. PMID: 19120092 Free PMC article. Review.
Cited by
- Intranasal oxytocin compensates for estrus cycle-specific reduction of conditioned safety memory in rats: Implications for psychiatric disorders.
Kreutzmann JC, Fendt M. Kreutzmann JC, et al. Neurobiol Stress. 2021 Mar 10;14:100313. doi: 10.1016/j.ynstr.2021.100313. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33778132 Free PMC article. - Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development.
Hostinar CE, Sullivan RM, Gunnar MR. Hostinar CE, et al. Psychol Bull. 2014 Jan;140(1):256-82. doi: 10.1037/a0032671. Epub 2013 Apr 22. Psychol Bull. 2014. PMID: 23607429 Free PMC article. Review. - Romantic Love vs. Drug Addiction May Inspire a New Treatment for Addiction.
Zou Z, Song H, Zhang Y, Zhang X. Zou Z, et al. Front Psychol. 2016 Sep 22;7:1436. doi: 10.3389/fpsyg.2016.01436. eCollection 2016. Front Psychol. 2016. PMID: 27713720 Free PMC article. Review. - Intranasal oxytocin may improve odds of abstinence in cocaine-dependent patients: results from a preliminary study.
Noël Raby W, Heller M, Milliaressis D, Jean Choi C, Basaraba C, Pavlicova M, Alschuler DM, Levin FR, Church S, Nunes EV. Noël Raby W, et al. Drug Alcohol Depend Rep. 2021 Dec 10;2:100016. doi: 10.1016/j.dadr.2021.100016. eCollection 2022 Mar. Drug Alcohol Depend Rep. 2021. PMID: 36845891 Free PMC article. - The effect of oxytocin and an enriched environment on anxiety-like behaviour and corticosterone levels in a prenatally stressed febrile seizure rat model.
Maikoo S, Wilkins A, Qulu L. Maikoo S, et al. IBRO Neurosci Rep. 2022 May 17;13:47-56. doi: 10.1016/j.ibneur.2022.05.001. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36590100 Free PMC article.
References
- Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW (1995) Suppression of the hypothalamic pituitary-adrenal axis responses to stress in lactating women. J Clin Invest 80: 2954–2959. - PubMed
- Antoni FA, Holmes MC, Jones MT (1983) Oxytocin as well as vasopressin potentiate ovine CRF in vitro. Peptides 4: 411–415. - PubMed
- Arnold FJL, De Lucas Bueno M, Shier H, Hancock DC, Evan GI, Herbert J (1992) Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience 51: 377–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources