Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade - PubMed (original) (raw)
Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade
Jean-Martin Beaulieu et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Dopamine (DA) is a neurotransmitter involved in the control of locomotion, emotion, cognition, and reward. Administration of lithium salts is known to inhibit DA-associated behaviors in experimental animal models through unknown mechanisms. Here, we used a pharmacogenetic approach to show that DA can exert its behavioral effects by acting on a lithium-sensitive signaling cascade involving Akt/PKB and glycogen synthase kinase 3 (GSK-3). In the mouse striatum, increased DA neurotransmission arising either from administration of amphetamine or from the lack of the DA transporter results in inactivation of Akt and concomitant activation of GSK-3alpha and GSK-3beta. These biochemical changes are not affected by activation of the cAMP pathway but are effectively reversed either by inhibition of DA synthesis, D2 receptor blockade, or administration of lithium salts. Furthermore, pharmacological or genetic inhibition of GSK-3 significantly reduces DA-dependent locomotor behaviors. These data support the involvement of GSK-3 as an important mediator of DA and lithium action in vivo and suggest that modulation of the Akt/GSK-3 pathway might be relevant to DA-related disorders, such as attention deficit hyperactivity disorder and schizophrenia.
Figures
Fig. 1.
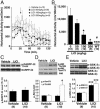
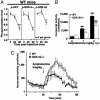
Lithium antagonizes behavioral responses to DA in DAT-KO mice. (A) DAT-KO mice were placed in a locomotor activity monitor for an initial period of 30 min and were then injected (arrow) with vehicle (saline) or with LiCl (50, 100, or 200 mg/kg of body weight i.p.). Horizontal activity was recorded every 5 min for a 2-h period. (B) Horizontal activity was quantified for a period of 1 h after injection of either vehicle or different doses of LiCl. Note that LiCl-treated DAT-KO mice remained more active than vehicle-treated WT mice observed under the same conditions. (_C_-E) Densitometric Western blot analysis of phosphoprotein relative levels in extract prepared from the striatum of DAT-KO mice 30 min after injection of LiCl (200 mg/kg of body weight i.p.) or vehicle. Antibodies directed against _p_-Thr-34-DARPP-32 (C), _p_-Thr-308-Akt (open bar in D), and _p_-Ser-473-Akt (filled bar in D) and with an antibody recognizing both _p_-GSK-3α (Ser-21, filled bar in E) and β (Ser-9, open bars in E) were used. Phospho-independent antibodies directed against these different proteins were used as loading controls for densitometry. For all results, data are means ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.001 as compared with vehicle-treated DAT-KO mice; #, P < 0.05 as compared with DAT-KO mice treated with 200 mg/kg LiCl. Numbers of animals per group (n) are indicated.
Fig. 2.
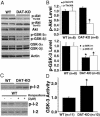
Inactivation of Akt and concomitant activation of GSK-3α and -3β in the striatum of DAT-KO mice. (A) Western blots showing the relative levels of total and phospho-Akt (Ser-473 and Thr-308) in the striatum of DAT-KO mice and WT littermates. (B) Densitometric analysis of the levels of _p_-Thr-308 (Upper, open bars), _p_-Ser-473-Akt (Upper, filled bars) and of phospho-GSK-3α and -3β (Lower) in mice from both genotypes. (C and D) GSK-3 activity assays performed after immunoprecipitation of striatal extracts by using a pan GSK-3α/β antibody. (C Upper) Autoradiogram showing the incorporation of 32P in recombinant phosphatase inhibitor 2 (I-2) by immunoprecipitates from WT and DAT-KO mice. (C Lower) Inhibition of kinase activity in the immunoprecipitates when the assay was performed in the presence of 2 μM GSK-3 inhibitor kenpaullone. Total inhibitor 2 was revealed by Coomassie blue staining. (D) Quantitative analysis revealed an ≈1.8-fold increase in GSK-3 activity in the striatum of DAT-KO mice. For densitometric analysis, optical densities obtained from specific phospho-independent antibodies were used as a loading control for the evaluation of phosphoprotein levels, and actin was used as a general loading control. Data are means ± SEM. *, P < 0.05 as compared with WT littermates.
Fig. 3.
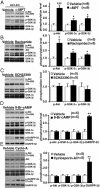
Regulation of Akt and GSK-3 by DA in DAT-KO (A) Western blots showing increased levels of _p_-Thr-308-Akt, _p_-GSK-3α, and _p_-GSK-3β in the striatum of DAT-KO mice 2 h after inhibition of DA synthesis by injection ofαMPT (250 mg/kg of body weight i.p.). DA depletion in the striatum under these conditions was confirmed with microdialysis (Fig. 9). Data are means ± SEM. *, P < 0.05; ***, P < 0.001. (B) The D2-class receptor antagonist raclopride blocks the action of DA on the phosphorylation of Akt (Thr-308), GSK-3α, and GSK-3β at 1 h after injection. (C) No effect of the D1-class receptor antagonist SCH23390 (0.1 mg/kg of body weight s.c.) on Akt and GSK-3α/β phosphorylation as evaluated by Western blot analysis. (D and E) Intracerebroventricular injection of 8-Br-cAMP (50 nmol) (D) or of cyclosporin A (4 μl of a 10-μM solution) (E) increased phosphorylation of DARPP-32 (Thr-34) without affecting Akt and GSK-3α/β phosphorylation at 30 min after injection. Data are means ± SEM. *, P < 0.05; **, P ≤ 0.005; ***, P ≤ 0.001 as compared with vehicle-treated DAT-KO mice.
Fig. 4.
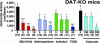
Inhibition of horizontal activity in DAT-KO mice by different GSK-3 inhibitors. DAT-KO mice were placed in a locomotor activity monitor for an initial period of 30 min and were then injected with vehicle or with SB 216763 (3, 5, or 10 mg/kg of body weight i.p.), Indirubin-3′-monoxime (5, 10, or 20 mg/kg of body weight i.p.), Alsterpaullone (3, 5, and 10 mg/kg of body weight i.p.), Sodium valproate (100, 200, or 300 mg/kg of body weight i.p.) or TDZD (30 mg/kg of body weight i.p.). All effects were monitored for a period of 30 min starting 5 min after injection, except for sodium valproate for which measurement started 45 min after injection, and compared with a separate set of vehicle-treated mice observed under the same conditions. Data are means ± SEM. *, P < 0.05; ***, P < 0.001 as compared with vehicle-treated mice. Numbers of animals per group (n) are indicated.
Fig. 5.
Involvement of GSK-3 in the action of amphetamine in vivo. (A) Quantification of Akt (Thr-308), GSK-3α, and GSK-3β phosphorylation as measured by Western blot analysis at 0, 30, and 90 min after amphetamine injection. *, P < 0.05; P < 0.005 as compared with saline-treated animals. (B and C) Reduced response to amphetamine in GSK-3β+/- mice. (B) Changes in horizontal activity induced by different doses of amphetamine (0, 1, or 2 mg/kg of body weight i.p., n = 6 mice per group) in GSK-3β+/- mice and WT littermates as measured for a period of 90 min after injection. (C) Locomotor activity curve of WT and GSK-3β+/- mice after injection of 2 mg of amphetamine/kg of body weight (i.p.). Data are means ± SEM. *, P < 0.05; **, P < 0.005 as compared with WT mice receiving the same dose of amphetamine; and ##, P < 0.005; ###, P < 0.001 as compared with vehicle-treated mice from the same genotype.
Fig. 6.
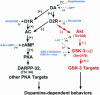
Working model of potential mechanisms of DA receptor signaling and sites of action of the different pharmacological agents used in this study. In red, regulation of the Akt/GSK-3 pathway by DA. Elevated DA tones lead to activation of GSK-3 through a signaling cascade involving D2-class receptors and the inactivation of Akt caused by a reduction of its phosphorylation on Thr-308. This reduction in Akt activity results in a reduced phosphorylation/increased activation of GSK-3α and GSK-3β that, in turn, regulate DA-associated behaviors. Note that, in contrast to DARPP-32, the phosphorylation of Akt and GSK-3 was not affected by cAMP. Dashed lines indicate the effect of different drugs on this signaling cascade. D1R, DA D1 receptor; D2R, DA D2 receptor; AC, adenylyl cyclase.
Similar articles
- The role of glycogen synthase kinase 3beta in insulin-stimulated glucose metabolism.
Summers SA, Kao AW, Kohn AD, Backus GS, Roth RA, Pessin JE, Birnbaum MJ. Summers SA, et al. J Biol Chem. 1999 Jun 18;274(25):17934-40. doi: 10.1074/jbc.274.25.17934. J Biol Chem. 1999. PMID: 10364240 - Glycogen synthase kinase-3beta heterozygote knockout mice as a model of findings in postmortem schizophrenia brain or as a model of behaviors mimicking lithium action: negative results.
Bersudsky Y, Shaldubina A, Kozlovsky N, Woodgett JR, Agam G, Belmaker RH. Bersudsky Y, et al. Behav Pharmacol. 2008 May;19(3):217-24. doi: 10.1097/FBP.0b013e3282feb099. Behav Pharmacol. 2008. PMID: 18469539 - Lithium-related genetics of bipolar disorder.
Detera-Wadleigh SD. Detera-Wadleigh SD. Ann Med. 2001 May;33(4):272-85. doi: 10.3109/07853890108998756. Ann Med. 2001. PMID: 11405549 Review. - The Akt-GSK-3 signaling cascade in the actions of dopamine.
Beaulieu JM, Gainetdinov RR, Caron MG. Beaulieu JM, et al. Trends Pharmacol Sci. 2007 Apr;28(4):166-72. doi: 10.1016/j.tips.2007.02.006. Epub 2007 Mar 8. Trends Pharmacol Sci. 2007. PMID: 17349698 Review.
Cited by
- Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins.
de Bartolomeis A, Buonaguro EF, Iasevoli F. de Bartolomeis A, et al. Psychopharmacology (Berl). 2013 Jan;225(1):1-19. doi: 10.1007/s00213-012-2921-8. Epub 2012 Nov 21. Psychopharmacology (Berl). 2013. PMID: 23179966 Review. - GSK3: A potential target and pending issues for treatment of Alzheimer's disease.
Zhao J, Wei M, Guo M, Wang M, Niu H, Xu T, Zhou Y. Zhao J, et al. CNS Neurosci Ther. 2024 Jul;30(7):e14818. doi: 10.1111/cns.14818. CNS Neurosci Ther. 2024. PMID: 38946682 Free PMC article. Review. - G protein-coupled receptor kinases as regulators of dopamine receptor functions.
Gurevich EV, Gainetdinov RR, Gurevich VV. Gurevich EV, et al. Pharmacol Res. 2016 Sep;111:1-16. doi: 10.1016/j.phrs.2016.05.010. Epub 2016 May 10. Pharmacol Res. 2016. PMID: 27178731 Free PMC article. Review. - GSK-3β: A Bifunctional Role in Cell Death Pathways.
Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. Jacobs KM, et al. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. Epub 2012 May 21. Int J Cell Biol. 2012. PMID: 22675363 Free PMC article. - Selective deletion of forebrain glycogen synthase kinase 3β reveals a central role in serotonin-sensitive anxiety and social behaviour.
Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Latapy C, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2460-74. doi: 10.1098/rstb.2012.0094. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826345 Free PMC article.
References
- Carlsson, A. (1987) Annu. Rev. Neurosci. 10, 19-40. - PubMed
- Greengard, P. (2001) Science 294, 1024-1030. - PubMed
- Gainetdinov, R. R. & Caron, M. G. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 261-284. - PubMed
- Giros, B., Jaber, M., Jones, S. R., Wightman, R. M. & Caron, M. G. (1996) Nature 379, 606-612. - PubMed
- Gainetdinov, R. R., Wetsel, W. C., Jones, S. R., Levin, E. D., Jaber, M. & Caron, M. G. (1999) Science 283, 397-401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous