Locked nucleic acid (LNA) single nucleotide polymorphism (SNP) genotype analysis and validation using real-time PCR - PubMed (original) (raw)
Locked nucleic acid (LNA) single nucleotide polymorphism (SNP) genotype analysis and validation using real-time PCR
Matthew P Johnson et al. Nucleic Acids Res. 2004.
Abstract
With an increased emphasis on genotyping of single nucleotide polymorphisms (SNPs) in disease association studies, the genotyping platform of choice is constantly evolving. In addition, the development of more specific SNP assays and appropriate genotype validation applications is becoming increasingly critical to elucidate ambiguous genotypes. In this study, we have used SNP specific Locked Nucleic Acid (LNA) hybridization probes on a real-time PCR platform to genotype an association cohort and propose three criteria to address ambiguous genotypes. Based on the kinetic properties of PCR amplification, the three criteria address PCR amplification efficiency, the net fluorescent difference between maximal and minimal fluorescent signals and the beginning of the exponential growth phase of the reaction. Initially observed SNP allelic discrimination curves were confirmed by DNA sequencing (n = 50) and application of our three genotype criteria corroborated both sequencing and observed real-time PCR results. In addition, the tested Caucasian association cohort was in Hardy-Weinberg equilibrium and observed allele frequencies were very similar to two independently tested Caucasian association cohorts for the same tested SNP. We present here a novel approach to effectively determine ambiguous genotypes generated from a real-time PCR platform. Application of our three novel criteria provides an easy to use semi-automated genotype confirmation protocol.
Figures
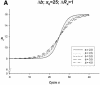
Figure 1
Simulated PCR phase transitions produced from SigmaPlot (version 8.0, SPSS) displaying amplification curves for a true heterozygous genotype. The theoretical behaviour of PCR amplification is determined by the initial ground (IG), exponential growth (EG), linear growth (LG) and plateau (P) phases, respectively. Based on a four-parameter sigmoid model (equation 1), the variables _y_0, a, _x_0 and b are obtained to determine ambiguous genotypes based on three criteria.
Figure 2
Simulation curves produced from SigmaPlot (version 8.0, SPSS) examining changes in PCR amplification efficiency (Δ_b_), net fluorescent signal (Δ_R_x) and the point of inflection—FDM (Δ_x_0). All simulations represent amplification on one fluorophore channel of the Rotor-Gene 3000™. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (R_x). (A) Changes in amplification efficiency (Δ_b) with a constant point of inflection (_x_0 = 25) and a constant net fluorescent signal (Δ_R_x = 1.0). (B) Changes in net fluorescent signal gains (Δ_R_x) with a constant point of inflection (x_0 = 25) and a constant PCR amplification efficiency (b = 2.0; E = 1.0). (C) Changes in amplification efficiency (Δ_b) and net fluorescent signal gains (Δ_R_x) with a constant point of inflection (_x_0 = 25). (D) Changes in the point of inflection (Δ_x_0) (FDM) with a constant amplification efficiency (b = 2.0; E = 1.0) and constant net fluorescence signal gain (Δ_R_x = 1.0).
Figure 2
Simulation curves produced from SigmaPlot (version 8.0, SPSS) examining changes in PCR amplification efficiency (Δ_b_), net fluorescent signal (Δ_R_x) and the point of inflection—FDM (Δ_x_0). All simulations represent amplification on one fluorophore channel of the Rotor-Gene 3000™. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (R_x). (A) Changes in amplification efficiency (Δ_b) with a constant point of inflection (_x_0 = 25) and a constant net fluorescent signal (Δ_R_x = 1.0). (B) Changes in net fluorescent signal gains (Δ_R_x) with a constant point of inflection (x_0 = 25) and a constant PCR amplification efficiency (b = 2.0; E = 1.0). (C) Changes in amplification efficiency (Δ_b) and net fluorescent signal gains (Δ_R_x) with a constant point of inflection (_x_0 = 25). (D) Changes in the point of inflection (Δ_x_0) (FDM) with a constant amplification efficiency (b = 2.0; E = 1.0) and constant net fluorescence signal gain (Δ_R_x = 1.0).
Figure 2
Simulation curves produced from SigmaPlot (version 8.0, SPSS) examining changes in PCR amplification efficiency (Δ_b_), net fluorescent signal (Δ_R_x) and the point of inflection—FDM (Δ_x_0). All simulations represent amplification on one fluorophore channel of the Rotor-Gene 3000™. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (R_x). (A) Changes in amplification efficiency (Δ_b) with a constant point of inflection (_x_0 = 25) and a constant net fluorescent signal (Δ_R_x = 1.0). (B) Changes in net fluorescent signal gains (Δ_R_x) with a constant point of inflection (x_0 = 25) and a constant PCR amplification efficiency (b = 2.0; E = 1.0). (C) Changes in amplification efficiency (Δ_b) and net fluorescent signal gains (Δ_R_x) with a constant point of inflection (_x_0 = 25). (D) Changes in the point of inflection (Δ_x_0) (FDM) with a constant amplification efficiency (b = 2.0; E = 1.0) and constant net fluorescence signal gain (Δ_R_x = 1.0).
Figure 2
Simulation curves produced from SigmaPlot (version 8.0, SPSS) examining changes in PCR amplification efficiency (Δ_b_), net fluorescent signal (Δ_R_x) and the point of inflection—FDM (Δ_x_0). All simulations represent amplification on one fluorophore channel of the Rotor-Gene 3000™. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (R_x). (A) Changes in amplification efficiency (Δ_b) with a constant point of inflection (_x_0 = 25) and a constant net fluorescent signal (Δ_R_x = 1.0). (B) Changes in net fluorescent signal gains (Δ_R_x) with a constant point of inflection (x_0 = 25) and a constant PCR amplification efficiency (b = 2.0; E = 1.0). (C) Changes in amplification efficiency (Δ_b) and net fluorescent signal gains (Δ_R_x) with a constant point of inflection (_x_0 = 25). (D) Changes in the point of inflection (Δ_x_0) (FDM) with a constant amplification efficiency (b = 2.0; E = 1.0) and constant net fluorescence signal gain (Δ_R_x = 1.0).
Figure 3
Allelic discrimination curves produced by the Rotor-Gene 3000™ analysis software. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (_R_x). (A) Example of a true heterozygote (TC) with the amplification curves for both fluorophore channels (JOE, FAM) satisfying the three proposed criteria. (B) Example of a true homozygote (CC) with the amplification curve of the JOE channel satisfying Criteria A and B. (C) Example of a true homozygote (TT) with the amplification of the other channel (FAM) satisfying Criteria A and B. (D) Example of an ambiguous heterozygote. Criteria A and B (allowing a 5% error) were not satisfied for the FAM fluorophore channel, and Criterion C was not met. Hence, a true homozygote (CC) is deduced. (E) Example of another (observational) ambiguous heterozygote. All three genotype criteria were not satisfied for both fluorophore channels (allowing a 5% error). Hence, this sample is excluded from genotypic and allelic association analyses.
Figure 3
Allelic discrimination curves produced by the Rotor-Gene 3000™ analysis software. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (_R_x). (A) Example of a true heterozygote (TC) with the amplification curves for both fluorophore channels (JOE, FAM) satisfying the three proposed criteria. (B) Example of a true homozygote (CC) with the amplification curve of the JOE channel satisfying Criteria A and B. (C) Example of a true homozygote (TT) with the amplification of the other channel (FAM) satisfying Criteria A and B. (D) Example of an ambiguous heterozygote. Criteria A and B (allowing a 5% error) were not satisfied for the FAM fluorophore channel, and Criterion C was not met. Hence, a true homozygote (CC) is deduced. (E) Example of another (observational) ambiguous heterozygote. All three genotype criteria were not satisfied for both fluorophore channels (allowing a 5% error). Hence, this sample is excluded from genotypic and allelic association analyses.
Figure 3
Allelic discrimination curves produced by the Rotor-Gene 3000™ analysis software. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (_R_x). (A) Example of a true heterozygote (TC) with the amplification curves for both fluorophore channels (JOE, FAM) satisfying the three proposed criteria. (B) Example of a true homozygote (CC) with the amplification curve of the JOE channel satisfying Criteria A and B. (C) Example of a true homozygote (TT) with the amplification of the other channel (FAM) satisfying Criteria A and B. (D) Example of an ambiguous heterozygote. Criteria A and B (allowing a 5% error) were not satisfied for the FAM fluorophore channel, and Criterion C was not met. Hence, a true homozygote (CC) is deduced. (E) Example of another (observational) ambiguous heterozygote. All three genotype criteria were not satisfied for both fluorophore channels (allowing a 5% error). Hence, this sample is excluded from genotypic and allelic association analyses.
Figure 3
Allelic discrimination curves produced by the Rotor-Gene 3000™ analysis software. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (_R_x). (A) Example of a true heterozygote (TC) with the amplification curves for both fluorophore channels (JOE, FAM) satisfying the three proposed criteria. (B) Example of a true homozygote (CC) with the amplification curve of the JOE channel satisfying Criteria A and B. (C) Example of a true homozygote (TT) with the amplification of the other channel (FAM) satisfying Criteria A and B. (D) Example of an ambiguous heterozygote. Criteria A and B (allowing a 5% error) were not satisfied for the FAM fluorophore channel, and Criterion C was not met. Hence, a true homozygote (CC) is deduced. (E) Example of another (observational) ambiguous heterozygote. All three genotype criteria were not satisfied for both fluorophore channels (allowing a 5% error). Hence, this sample is excluded from genotypic and allelic association analyses.
Figure 3
Allelic discrimination curves produced by the Rotor-Gene 3000™ analysis software. The _x_-axis represents the amplification cycle number and on the _y_-axis is the raw fluorescent value (_R_x). (A) Example of a true heterozygote (TC) with the amplification curves for both fluorophore channels (JOE, FAM) satisfying the three proposed criteria. (B) Example of a true homozygote (CC) with the amplification curve of the JOE channel satisfying Criteria A and B. (C) Example of a true homozygote (TT) with the amplification of the other channel (FAM) satisfying Criteria A and B. (D) Example of an ambiguous heterozygote. Criteria A and B (allowing a 5% error) were not satisfied for the FAM fluorophore channel, and Criterion C was not met. Hence, a true homozygote (CC) is deduced. (E) Example of another (observational) ambiguous heterozygote. All three genotype criteria were not satisfied for both fluorophore channels (allowing a 5% error). Hence, this sample is excluded from genotypic and allelic association analyses.
Similar articles
- Enhanced allele-specific PCR discrimination in SNP genotyping using 3' locked nucleic acid (LNA) primers.
Latorra D, Campbell K, Wolter A, Hurley JM. Latorra D, et al. Hum Mutat. 2003 Jul;22(1):79-85. doi: 10.1002/humu.10228. Hum Mutat. 2003. PMID: 12815597 - Single nucleotide polymorphism genotyping using locked nucleic acid (LNA).
Mouritzen P, Nielsen AT, Pfundheller HM, Choleva Y, Kongsbak L, Møller S. Mouritzen P, et al. Expert Rev Mol Diagn. 2003 Jan;3(1):27-38. doi: 10.1586/14737159.3.1.27. Expert Rev Mol Diagn. 2003. PMID: 12528362 Review. - Single-nucleotide-polymorphism-specific PCR for quantification and discrimination of Chlamydia pneumoniae genotypes by use of a "locked" nucleic acid.
Rupp J, Solbach W, Gieffers J. Rupp J, et al. Appl Environ Microbiol. 2006 May;72(5):3785-7. doi: 10.1128/AEM.72.5.3785-3787.2006. Appl Environ Microbiol. 2006. PMID: 16672536 Free PMC article. - Genotyping of the triallelic variant G2677T/A in MDR1 using LightCycler with locked-nucleic-acid-modified hybridization probes.
Arjomand-Nahad F, Diefenbach K, Landt O, Gaikovitch E, Roots I. Arjomand-Nahad F, et al. Anal Biochem. 2004 Nov 1;334(1):201-3. doi: 10.1016/j.ab.2004.07.030. Anal Biochem. 2004. PMID: 15464971 No abstract available. - The use of real-time PCR methods in DNA sequence variation analysis.
Gibson NJ. Gibson NJ. Clin Chim Acta. 2006 Jan;363(1-2):32-47. doi: 10.1016/j.cccn.2005.06.022. Epub 2005 Sep 22. Clin Chim Acta. 2006. PMID: 16182268 Review.
Cited by
- Mapping the nicking efficiencies of nickase R.BbvCI for side-specific LNA-substituted substrates using rolling circle amplification.
Wei H, Zhao G, Hu T, Tang S, Jiang J, Hu B, Guan Y. Wei H, et al. Sci Rep. 2016 Sep 1;6:32560. doi: 10.1038/srep32560. Sci Rep. 2016. PMID: 27582033 Free PMC article. - Specific detection and real-time PCR quantification of potentially mycophagous bacteria belonging to the genus Collimonas in different soil ecosystems.
Höppener-Ogawa S, Leveau JH, Smant W, van Veen JA, de Boer W. Höppener-Ogawa S, et al. Appl Environ Microbiol. 2007 Jul;73(13):4191-7. doi: 10.1128/AEM.00387-07. Epub 2007 May 4. Appl Environ Microbiol. 2007. PMID: 17483278 Free PMC article. - Development of a Molecular Assay for Detection and Quantification of the BRAF Variation in Residual Tissue From Thyroid Nodule Fine-Needle Aspiration Biopsy Specimens.
Fu G, Chazen RS, MacMillan C, Witterick IJ. Fu G, et al. JAMA Netw Open. 2021 Oct 1;4(10):e2127243. doi: 10.1001/jamanetworkopen.2021.27243. JAMA Netw Open. 2021. PMID: 34613404 Free PMC article. - Evaluation of control transcripts in real-time RT-PCR expression analysis during maritime pine embryogenesis.
Gonçalves S, Cairney J, Maroco J, Oliveira MM, Miguel C. Gonçalves S, et al. Planta. 2005 Oct;222(3):556-63. doi: 10.1007/s00425-005-1562-0. Epub 2005 Jul 21. Planta. 2005. PMID: 16034587 - Oligonucleotide microarray for the identification of potential mycotoxigenic fungi.
Lezar S, Barros E. Lezar S, et al. BMC Microbiol. 2010 Mar 23;10:87. doi: 10.1186/1471-2180-10-87. BMC Microbiol. 2010. PMID: 20307326 Free PMC article.
References
- Tsuchihashi Z. and Dracopoli,N.C. (2002) Progress in high throughput SNP genotyping methods. Pharmacogenomics J., 2, 103–110. - PubMed
- Syvanen A.C. (2001) Accessing genetic variation: genotyping single nucleotide polymorphisms. Nat. Rev. Genet., 2, 930–942. - PubMed
- Shi M.M. (2001) Enabling large-scale pharmacogenetic studies by high-throughput mutation detection and genotyping technologies. Clin. Chem., 47, 164–172. - PubMed
- Petersen M. and Wengel,J. (2003) LNA: a versatile tool for therapeutics and genomics. Trends Biotechnol., 21, 74–81. - PubMed
- Braasch D.A. and Corey,D.R. (2001) Locked nucleic acid (LNA): fine-tuning the recognition of DNA and RNA. Chem. Biol., 8, 1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources