Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins - PubMed (original) (raw)
Comparative Study
Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins
Andrew A Peden et al. J Cell Biol. 2004.
Abstract
The adaptor protein (AP) 3 adaptor complex has been implicated in the transport of lysosomal membrane proteins, but its precise site of action has remained controversial. Here, we show by immuno-electron microscopy that AP-3 is associated with budding profiles evolving from a tubular endosomal compartment that also exhibits budding profiles positive for AP-1. AP-3 colocalizes with clathrin, but to a lesser extent than does AP-1. The AP-3- and AP-1-bearing tubular compartments contain endocytosed transferrin, transferrin receptor, asialoglycoprotein receptor, and low amounts of the cation-independent mannose 6-phosphate receptor and the lysosome-associated membrane proteins (LAMPs) 1 and 2. Quantitative analysis revealed that of these distinct cargo proteins, only LAMP-1 and LAMP-2 are concentrated in the AP-3-positive membrane domains. Moreover, recycling of endocytosed LAMP-1 and CD63 back to the cell surface is greatly increased in AP-3-deficient cells. Based on these data, we propose that AP-3 defines a novel pathway by which lysosomal membrane proteins are transported from tubular sorting endosomes to lysosomes.
Figures
Figure 1.
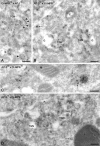
AP-3 is not found in the TGN of HepG2 cells. (A) In HepG2 cells, CI-MPR (10-nm gold) and AP-1 (15-nm gold) colocalize on membranes at the trans-side of the Golgi complex, indicating the position of the TGN. Some TGN membranes typically contain elongated stretches of clathrin (top row of arrowheads). The AP-1– and CI-MPR–labeled budding profiles in the TGN (bottom two arrowheads) are considerably wider than AP-3–labeled profiles (B–D, arrows). (B–D) AP-3 (15-nm gold) is not found on membranes in the TGN area, but on tubulo-vesicular profiles dispersed in the cytoplasm (B and D, arrows) or near endosomal vacuoles (C, arrows). E, endosome; G, Golgi complex; L, lysosome; M, mitochondrion; P, plasma membrane. Bars, 200 nm.
Figure 2.
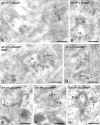
Ultrathin cryosections of HepG2 cells showing the presence of AP-3 on membrane buds evolving from EE-associated tubules. (A and B) HepG2 cells were incubated with Tf-biotin for 20 min to define early endosomes (EEs). Sections were double labeled for AP-3 (15-nm gold) and biotin (10-nm gold). AP-3 colocalizes with Tf-biotin in the recycling tubules (arrows) that emerge from the EE vacuoles. Note that AP-3 labeling is restricted to tubular endings and budding profiles on the tubules. (C) HepG2 cells were loaded for 60 min with BSA 5-nm gold, which marks the lysosomal pathway and is absent from recycling tubules. AP-3 (15-nm gold) is found on recycling tubules containing ASGPR (10-nm gold, arrows). (D) Labeling as in C, but in the absence of internalized BSA-gold. AP-3 is localized to buds that evolve from EE-associated recycling tubules containing ASGPR (arrows). (E–G) AP-3 (15-nm gold) and CI-MPR (10-nm gold). (E) Low labeling densities of CI-MPR are present in endosome (E)-associated tubules with AP-3–positive buds (arrow). (F and G) Occasionally, CI-MPR label is seen concentrated in budding profiles (arrowheads) that are not labeled for AP-3 (arrows). Bars, 200 nm.
Figure 3.
AP-3–positive buds partially colocalize with clathrin, whereas AP-3 and AP-1 localize to distinct buds evolving from a single endosomal tubule. (A and B) HepG2 cells double labeled for AP-3 (10-nm gold) and clathrin (15-nm gold). Arrows point to AP-3–positive buds that are also stained for clathrin. Open arrowheads mark membrane profiles labeled for AP-3 only. Closed arrowheads denote clathrin labeling only. (C) HepG2 cells double labeled for AP-3 (15-nm gold, arrow) and AP-1 (10-nm gold, arrowhead), showing that the two adaptor complexes are present on distinct buds of a continuous tubule. (D) Similar labeling as in A, but in addition the presence of internalized Tf-biotin (for 20 min at 37°C) is indicated by 5-nm gold, unequivocally defining the endosomal origin of this tubule. (E) NRK cells. Labeling as in A. Bars, 200 nm.
Figure 4.
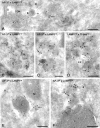
LAMP-1 is concentrated in AP-3–positive budding profiles. (A–C) Double labelings of AP-3 (15-nm gold) and LAMP-1 (10-nm gold), showing colocalization in endosome (E)-associated tubular–vesicular profiles (arrows). Low labeling densities of LAMP-1 are present in the AP-3–positive tubules. Note that in A, the LAMP-1/AP-3 positive tubule (arrow) has a smaller diameter than the typical TGN-associated clathrin-coated buds (arrowheads). (D) Colocalization of LAMP-1 (10-nm gold) and Tf-biotin internalized for 20 min (15-nm gold) unequivocally shows that LAMP-1 is present in EE-derived recycling tubules (arrows). (E and F) Double labelings of AP-3 (15-nm gold) and LAMP-1 (10-nm gold), showing that LAMP-1 is found within the AP-3–positive regions of the endosome-derived tubules (arrows). EE, early endosome; G, Golgi complex; L, lysosome; M, mitochondrion; N, nucleous; P, plasma membrane. Bars, 200 nm.
Figure 5.
LAMP-1 concentrates in AP-3–coated buds on ASGPR-positive tubular endosomes. (A–C) HepG2 cells triple labeled for AP-3 (15-nm gold), LAMP-1 (10-nm gold), and ASGPR (5-nm gold). In A, note the extensive network of endosome (E)-associated tubules (arrowhead) that labels positive for all three proteins. In B and C, the arrows point to AP-3–positive parts of the ASGPR-labeled tubules that contain LAMP-1. Bars, 200 nm.
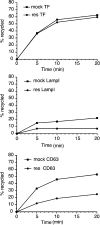
Figure 6.
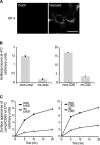
AP-3 deficiency leads to increased levels of LAMP-1 and CD63 on the cell surface. mocha cells expressing human CD63 were infected with either empty virus (mock) or virus expressing the δ subunit of the AP-3 complex (rescued). (A) After selection, >95% of the mocha cells was rescued. Bar, 30 μm. (B) Mock and rescued cells were incubated with either anti-LAMP-1 or anti-CD63 antibodies at 4°C. The steady-state levels of LAMP-1 and CD63 at the cell surface are greatly increased in AP-3–deficient cells, as determined by flow cytometry. (C) Mock and rescued cells were incubated for 20 min with either anti-LAMP-1 or anti-CD63 antibodies at 37°C. The delivery of LAMP-1 and CD63 to the cells surface is greatly decreased in rescued cells, as determined by flow cytometry. See also Fig. S3 (available at
http://www.jcb.org/cgi/content/full/jcb.200311064/DC1
).
Figure 7.
AP-3 deficiency leads to the increased recycling of lysosomal membrane proteins via the cell surface. Mock and rescued cells were allowed to internalize antibodies against LAMP-1, CD63, and TfR for 20 min at 37°C. The cells were washed and the amount of recycling was determined by incubating the cells for various amounts of time at 37°C in the presence of quenching antibody (anti-Alexa® 488). The cells were then analyzed by flow cytometry. The degree of recycling of LAMP-1 and CD63 is greatly enhanced in AP-3–deficient cells (mock); however, recycling of the TfR is unaffected. See also Fig. S4 (available at
http://www.jcb.org/cgi/content/full/jcb.200311064/DC1
).
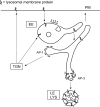
Figure 8.
Proposed role for AP-3 in the trafficking of lysosomal membrane proteins. Lysosomal membrane proteins reach EE vacuoles (or sorting endosomes) directly from the TGN or from an indirect pathway via the plasma membrane. Proteins that are not incorporated into the internal endosomal vesicles will by default enter the tubular extensions that emerge from the endosomal vacuole. The default pathway from the tubular endosomes is to the plasma membrane, but there is also an AP-1– and AP-3–mediated exit. The AP-3–containing membranes selectively concentrate lysosomal membrane proteins rather than proteins destined for TGN or plasma membrane, thus we propose they mediate transport to late endosomes/lysosomes. In this model, if AP-3 function were impaired, it would lead to an increased incorporation of lysosomal membrane proteins in the default recycling pathway, resulting in increased passage of lysosomal membrane proteins over the plasma membrane. E, early endosome; LE, late endosome; LYS, lysosome; PM, plasma membrane.
Similar articles
- The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles.
Höning S, Griffith J, Geuze HJ, Hunziker W. Höning S, et al. EMBO J. 1996 Oct 1;15(19):5230-9. EMBO J. 1996. PMID: 8895568 Free PMC article. - The role of the AP-1 adaptor complex in outgoing and incoming membrane traffic.
Robinson MS, Antrobus R, Sanger A, Davies AK, Gershlick DC. Robinson MS, et al. J Cell Biol. 2024 Jul 1;223(7):e202310071. doi: 10.1083/jcb.202310071. Epub 2024 Apr 5. J Cell Biol. 2024. PMID: 38578286 Free PMC article. - Differential use of two AP-3-mediated pathways by lysosomal membrane proteins.
Ihrke G, Kyttälä A, Russell MR, Rous BA, Luzio JP. Ihrke G, et al. Traffic. 2004 Dec;5(12):946-62. doi: 10.1111/j.1600-0854.2004.00236.x. Traffic. 2004. PMID: 15522097 - Clathrin and adaptors.
Hirst J, Robinson MS. Hirst J, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):173-93. doi: 10.1016/s0167-4889(98)00056-1. Biochim Biophys Acta. 1998. PMID: 9714795 Review. - New directions for the clathrin adaptor AP-1 in cell biology and human disease.
Duncan MC. Duncan MC. Curr Opin Cell Biol. 2022 Jun;76:102079. doi: 10.1016/j.ceb.2022.102079. Epub 2022 Apr 13. Curr Opin Cell Biol. 2022. PMID: 35429729 Free PMC article. Review.
Cited by
- Neurodegenerative VPS41 variants inhibit HOPS function and mTORC1-dependent TFEB/TFE3 regulation.
van der Welle REN, Jobling R, Burns C, Sanza P, van der Beek JA, Fasano A, Chen L, Zwartkruis FJ, Zwakenberg S, Griffin EF, Ten Brink C, Veenendaal T, Liv N, van Ravenswaaij-Arts CMA, Lemmink HH, Pfundt R, Blaser S, Sepulveda C, Lozano AM, Yoon G, Santiago-Sim T, Asensio CS, Caldwell GA, Caldwell KA, Chitayat D, Klumperman J. van der Welle REN, et al. EMBO Mol Med. 2021 May 7;13(5):e13258. doi: 10.15252/emmm.202013258. Epub 2021 Apr 14. EMBO Mol Med. 2021. PMID: 33851776 Free PMC article. - BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles.
Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. Bultema JJ, et al. J Biol Chem. 2012 Jun 1;287(23):19550-63. doi: 10.1074/jbc.M112.351908. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511774 Free PMC article. - AP-3 and Rabip4' coordinately regulate spatial distribution of lysosomes.
Ivan V, Martinez-Sanchez E, Sima LE, Oorschot V, Klumperman J, Petrescu SM, van der Sluijs P. Ivan V, et al. PLoS One. 2012;7(10):e48142. doi: 10.1371/journal.pone.0048142. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144738 Free PMC article. - Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins.
Melo RC, Spencer LA, Dvorak AM, Weller PF. Melo RC, et al. J Leukoc Biol. 2008 Feb;83(2):229-36. doi: 10.1189/jlb.0707503. Epub 2007 Sep 17. J Leukoc Biol. 2008. PMID: 17875811 Free PMC article. Review. - Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane.
Thomas DD, Martin CL, Weng N, Byrne JA, Groblewski GE. Thomas DD, et al. Am J Physiol Cell Physiol. 2010 Mar;298(3):C725-39. doi: 10.1152/ajpcell.00455.2009. Epub 2009 Dec 23. Am J Physiol Cell Physiol. 2010. PMID: 20032513 Free PMC article.
References
- Akasaki, K., M. Fukuzawa, H. Kinoshita, K. Furuno, and H. Tsuji. 1993. Cycling of two endogenous lysosomal membrane proteins, lamp-2 and acid phosphatase, between the cell surface and lysosomes in cultured rat hepatocytes. J. Biochem. (Tokyo). 114:598–604. - PubMed
- Akasaki, K., A. Michihara, K. Mibuka, Y. Fujiwara, and H. Tsuji. 1995. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp. Cell Res. 220:464–473. - PubMed
- Akasaki, K., A. Michihara, Y. Fujiwara, K. Mibuka, and H. Tsuji. 1996. Biosynthetic transport of a major lysosome-associated membrane glycoprotein 2, lamp-2: a significant fraction of newly synthesized lamp-2 is delivered to lysosomes by way of early endosomes. J. Biochem. (Tokyo). 120:1088–1094. - PubMed
- Clark, R.H., J.C. Stinchcombe, A. Day, E. Blott, S. Booth, G. Bossi, T. Hamblin, E.G. Davies, and G.M. Griffiths. 2003. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat. Immunol. 4:1111–1120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous