Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells - PubMed (original) (raw)
Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells
Petra Wolint et al. J Exp Med. 2004.
Abstract
CD8+ T cells play a central role in the resolution and containment of viral infections. A key effector function of CD8+ T cells is their cytolytic activity toward infected cells. Here, we studied the regulation of cytolytic activity in naive, effector, and central versus effector memory CD8+ T cells specific for the same glycoprotein-derived epitope of lymphocytic choriomeningitis virus. Our results show that the kinetics of degranulation, assessed by a novel flow cytometric based assay, were identical in effector and both subsets of memory CD8+ T cells, but absent in naive CD8+ T cells. However, immediate cytolytic activity was most pronounced in effector T cells, low in effector memory T cells, and absent in central memory T cells, correlating with the respective levels of cytolytic effector molecules present in lytic granules. These results indicate that an inherent program of degranulation is a feature of antigen-experienced cells as opposed to naive CD8+ T cells and that the ability of CD8+ T cells to induce target cell apoptosis/death is dependent on granule protein content rather than on the act of degranulation itself. Furthermore, these results provide a potential mechanism by which central memory CD8+ T cell-mediated death of antigen-presenting cells within the lymph node is avoided.
Figures
Figure 1.
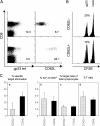
Kinetics of cell surface expression of CD107 and production of intracellular IFNγ. Spleen cells of acutely infected mice (“Effector,” day 8 after i.v. infection with 200 pfu LCMV WE) or memory mice (50 d after i.v. infection with 200 pfu LCMV WE) were incubated with 1 μg/ml gp33 peptide. At the indicated time points, surface expression of CD107a and the intracellular content of IFNγ was analyzed by flow cytometry. Plots are gated on CD8+ gp33 tetramer+ T cells. One representative staining of at least six different experiments is shown.
Figure 2.
Kinetics of cytotoxic activity of effector and memory gp33-specific T cells. Cytotoxic activity of spleen effector and memory CD8+ T cells was analyzed by 51Cr release assay. EL4 cells, pulsed with gp33 peptide or unpulsed, were used as target cells. Specific lysis was determined at the indicated time points of incubation. E/T ratios indicate the ratio between gp33 tetramer positive T cells and target cells. One of four similar experiments is shown.
Figure 3.
Kinetics degranulation and grB content of naive, effector, and memory gp33-specific T cells. (A) Spleen cells from memory (d50), acutely infected, or naive P14 transgenic mice were stimulated with 1 μg/ml gp33 peptide. At the indicated time points, cell surface expression of CD107a and intracellular grB content was analyzed by flow cytometry. Plots are gated on CD8+ gp33 tetramer+ T cells. One of three similar experiments is shown. (B) Acquisition of cell surface CD107 and changes in grB content in stimulated effector CD8+ T cells. The plots 1–4 show the percentage of CD8+ tet+ T cells present in the four quadrants (1, CD107+grB−; 2, CD107+grB+; 3, CD107−grB−; and 4, CD107−grB+) after different time points of stimulation.
Figure 4.
Cell surface expression of CD107a, intracellular IFNγ production, and grB content was analyzed at the indicated time points in effector and memory (d50) gp33-specific CD8+ T cells in the presence or absence of CHX. Plots are gated on CD8+ gp33 tetramer+ T cells. One of two equivalent experiments is shown.
Figure 5.
Degranulation and surface expression of CD62L and CD49d of central and effector gp33-specific memory T cells. (A) CD62L positive and CD62L negative cells were sorted from the spleen of LCMV memory mice (day 50) and stimulated with 1 μg/ml gp33 peptide. After the indicated time points, cell surface expression of CD107a and CD62L was analyzed by flow cytometry. Plots are gated on CD8+ gp33 tetramer+ T cells. One of three similar experiments is shown. (B) CD62L positive and CD62L negative cells were sorted from the spleen of LCMV memory mice (day 50) and stimulated with 1 μg/ml gp33 peptide. After the indicated time points, cell surface expression of CD107a and CD49d was analyzed by flow cytometry. Plots are either gated on CD8+ gp33 tetramer+ T cells or on CD8+ T cells. One of three similar experiments is shown. (C) Cell surface versus intracellular staining of CD107a. CD62L positive and CD62L negative cells were sorted from the spleen of LCMV memory mice (day 50). Sorted memory cells and day 8 effector cells were stained extracellularly or intracellularly for CD107a. Plots are gated on CD8+ T cells. One of two similar experiments is shown.
Figure 6.
Kinetics of degranulation, IFNγ production, and cytotoxicity of gp33-specific central and effector memory T cells. (A) Tetramer staining, cell surface CD107a expression, and intracellular IFNγ production was analyzed at the indicated time points in sorted CD62L positive and negative cells from LCMV memory mice (day 50). The top row shows staining of CD62L+ T cells (day 50) for CD8 and gp33 tetramer after the indicated time points of stimulation. The two bottom rows show staining for cell surface CD107 and intracellular IFNγ. Plots are gated on CD8+ gp33 tetramer+ T cells. One of three similar experiments is shown. (B) Cytotoxic activity of sorted CD62L positive and CD62L negative spleen cells of memory mice (day 50) was analyzed by 51Cr release assay. EL4 cells, pulsed with gp33 peptide or unpulsed were used as target cells. Specific lysis was determined at the indicated time points of incubation. E/T ratios indicate the ratio between gp33 tetramer positive T cells and target cells. The graphs on the right side show the percentage of grB+CD107− and CD107a+ cells of CD8+tet+ T cells at the indicated time points after stimulation. One of three similar experiments is shown. (C) Percentage of grB+ cells of CD8+ tet+ cells. grB positive cells were quantified before antigen stimulation amongst CD8+tet+ T cells of d8 effector mice, of memory mice (days 35 and 50), of sorted CD62L+ and CD62L− memory cells (days 30, 50, and 115), and of naive TCR transgenic mice.
Figure 7.
In vivo cytotoxicity of acutely LCMV-infected mice and memory mice. (A) gp33 peptide–loaded and unloaded target cells were adoptively transferred into acutely infected or memory recipient mice. Percentage of specific target cell elimination was analyzed in the blood at the indicated time points. (B) Specific target cell elimination was quantified in blood, spleen, and LN at 2 and 6 h after adoptive transfer of target cells. Numbers indicate the percentage of gp33 tetramer positive lymphocytes in the respective organs. (C) E/T ratio in blood, spleen, and LN at 2 and 6 h after adoptive transfer of target cells. Effector cells were gp33 tetramer positive lymphocytes. (D) Percentage of CD8+gp33 tetramer+CD62L+ cells in blood, spleen, and LN at 2 and 6 h after adoptive transfer of target cells. One of two similar experiments is shown.
Figure 8.
In vivo cytotoxicity of adoptively transfused gp33-specific central and effector memory cells. (A) CD62L positive and negative CD8 T cells were purified from d35 LCMV memory mice. (left) gp33 tetramer staining: numbers indicate the percentage of CD8+ tet+ T cells. (right) CD62L expression on tetramer-gated CD8+ T cells. (B) Target cell numbers in LN 4 h after transfer. 3 × 105 CD62L positive or CD62L negative CD8+tet+ T cells were transferred into naive recipient mice followed by transfer of 5 × 106 gp33-pulsed and mock-pulsed CFSE/PKH26 stained target cells 3 h later. CFSElow cells were pulsed with gp33 peptide and CFSEhigh cells were mock pulsed. 4 h after target cell transfer, the ratio of CFSElow/high target cells was analyzed in the LN. One representative staining of each group is shown. Cells are gated on PKH26 postive cells. Numbers indicate the percentage of specific target cell elimination. (C) Specific target cell elimination (a) 4 h after target cell transfer into recipients having been transfused with equal numbers of CD62L positive of CD62L negative LCMV memory T cells. (b) Percentage of CD8+ tet+ T cells 4 h after target cell transfer. (c) Percentage of PKH26+ target cells 4 h after target cell transfer. (d) E/T ratio (E = percentage of tetramer+ lymphocytes; T = percentage of PKH26+ lymphocytes) 4 h after target cell transfer.
Similar articles
- CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors.
Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, Ellmeier W, von Herrath MG, Jensen P, Littman DR, Cheroutre H. Madakamutil LT, et al. Science. 2004 Apr 23;304(5670):590-3. doi: 10.1126/science.1092316. Science. 2004. PMID: 15105501 - Phenotypic characteristics associated with the acquisition of HSV-specific CD8 T-lymphocyte-mediated cytolytic function in vitro.
McNally JM, Andersen HA, Chervenak R, Jennings SR. McNally JM, et al. Cell Immunol. 1999 May 25;194(1):103-11. doi: 10.1006/cimm.1999.1498. Cell Immunol. 1999. PMID: 10357886 - Characterization of naïve, memory and effector CD8+ T cells: effect of age.
Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Gupta S, et al. Exp Gerontol. 2004 Apr;39(4):545-50. doi: 10.1016/j.exger.2003.08.013. Exp Gerontol. 2004. PMID: 15050289 Review. - CD8 T cell dysfunction during chronic viral infection.
Shin H, Wherry EJ. Shin H, et al. Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review.
Cited by
- The emerging role of effector functions exerted by tissue-resident memory T cells.
Iijima N. Iijima N. Oxf Open Immunol. 2024 Jun 14;5(1):iqae006. doi: 10.1093/oxfimm/iqae006. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193473 Free PMC article. Review. - Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients' Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays.
Schutti O, Klauer L, Baudrexler T, Burkert F, Schmohl J, Hentrich M, Bojko P, Kraemer D, Rank A, Schmid C, Schmetzer H. Schutti O, et al. Int J Mol Sci. 2024 Jun 26;25(13):6983. doi: 10.3390/ijms25136983. Int J Mol Sci. 2024. PMID: 39000091 Free PMC article. - Cbl-b mitigates the responsiveness of naive CD8+ T cells that experience extensive tonic T cell receptor signaling.
Eggert J, Zinzow-Kramer WM, Hu Y, Kolawole EM, Tsai YL, Weiss A, Evavold BD, Salaita K, Scharer CD, Au-Yeung BB. Eggert J, et al. Sci Signal. 2024 Feb 6;17(822):eadh0439. doi: 10.1126/scisignal.adh0439. Epub 2024 Feb 6. Sci Signal. 2024. PMID: 38319998 Free PMC article. - Enhanced detection of antigen-specific T cells by a multiplexed AIM assay.
Lemieux A, Sannier G, Nicolas A, Nayrac M, Delgado GG, Cloutier R, Brassard N, Laporte M, Duchesne M, Sreng Flores AM, Finzi A, Tastet O, Dubé M, Kaufmann DE. Lemieux A, et al. Cell Rep Methods. 2024 Jan 22;4(1):100690. doi: 10.1016/j.crmeth.2023.100690. Epub 2024 Jan 15. Cell Rep Methods. 2024. PMID: 38228152 Free PMC article. - Characteristics of circulating immune cells in HBV-related acute-on-chronic liver failure following artificial liver treatment.
Ju T, Jiang D, Zhong C, Zhang H, Huang Y, Zhu C, Yang S, Yan D. Ju T, et al. BMC Immunol. 2023 Nov 25;24(1):47. doi: 10.1186/s12865-023-00579-8. BMC Immunol. 2023. PMID: 38007423 Free PMC article.
References
- van Stipdonk, M.J., E.E. Lemmens, and S.P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423–429. - PubMed
- Bachmann, M.F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29:291–299. - PubMed
- Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. - PubMed
- Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous