Reconstruction of functionally normal and malignant human breast tissues in mice - PubMed (original) (raw)
Reconstruction of functionally normal and malignant human breast tissues in mice
Charlotte Kuperwasser et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The study of normal breast epithelial morphogenesis and carcinogenesis in vivo has largely used rodent models. Efforts at studying mammary morphogenesis and cancer with xenotransplanted human epithelial cells have failed to recapitulate the full extent of development seen in the human breast. We have developed an orthotopic xenograft model in which both the stromal and epithelial components of the reconstructed mammary gland are of human origin. Genetic modification of human stromal cells before the implantation of ostensibly normal human mammary epithelial cells resulted in the outgrowth of benign and malignant lesions. This experimental model allows for studies of human epithelial morphogenesis and differentiation in vivo and underscores the critical role of heterotypic interactions in human breast development and carcinogenesis.
Figures
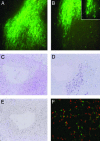
Fig. 1.
GFP whole-mount and histological analysis of human-murine chimeric fat pads. (A and B) GFP whole-mount examination of cleared mouse mammary fat pads injected with immortalized human breast fibroblasts 4 weeks postinjection (×10). Single GFP-positive cells can be seen infiltrating the adipose stroma (B Inset). (C) Hematoxylin/eosin-stained section of an area of fibroblastic cord growth within the cleared fat pad. (D and E) Serial sections of this region were examined by immunohistochemistry by using antibodies against human-specific vimentin (purple, D) or PCNA (brown, E). Genomic FISH was performed by using specific probes for mouse Cot I gene (red) and human genomic DNA (green) (F).
Fig. 2.
Whole-mount analysis of breast tissue of human breast and xenografted outgrowths. Normal human breast tissue isolated from reduction mammoplasty was stained with hematoxylin and whole-mounted exhibits both ductal and TDLU (in brackets) structures (A). Carmine-stained whole-mount examination of outgrowth from xenografts in humanized fat pads contain both ductal (B) and TDLU structures (C and D). Arrows point out commonly observed acini structures with hollow lumina. TDLU formation occurred during murine puberty (C) or pregnancy (D). Some outgrowth displayed evidence of both ductal and TDLU-like growths (E).
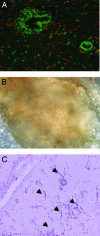
Fig. 3.
Epithelial and stromal cell contribution in xenograft breast tissue. Genomic FISH was performed on sections from mice in which human breast epithelial cells were injected into the humanized fat pads along with normal primary breast fibroblasts (A). Human cells (green) comprise all of the epithelial cells in the xenografts, but the stroma is comprised of both human and mouse cells (red). A significant amount of angiogenesis is observed within the xenograft (B). White-light photomicrograph of the xenografted implanted region 8 weeks after surgery displays significant infiltration of blood vessels. (C) Hematoxylin/eosin-stained section of a region of the xenograft reveals many capillaries (arrows) within the stroma indicative of neovascularization.
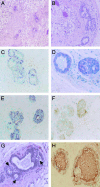
Fig. 4.
Histological and molecular analysis of xenograft breast tissues. Paraffin sections of breast xenograft ductal outgrowths were stained with hematoxylin/eosin (A and B). Double labeling of breast markers ductal structures (C). Myoepithelial cell-specific expression of smooth-muscle actin (purple) is not detected in cells expressing the estrogen receptor (brown). Immunohistochemistry was performed on serial sections with antibodies specific for PCNA (D) to demonstrate the proliferative state of the outgrowth. Brown nuclei can be detected in both epithelial and stromal cells. Luminal-specific expression of cytokeratin 19 is detected in ER expressing cells (E), and E-cadherin expression is detected on the membrane of luminal epithelial cells (F). Paraffin sections of fully differentiated breast xenograft ductal outgrowths in pregnant mice were stained with hematoxylin and eosin (G). Milk protein and fat droplets are seen (arrows) in the differentiated epithelial cells under the control of the hormones of pregnancy. Immunohistochemistry was performed on these sections with antibodies against human-specific β-casein milk protein (H). β-Casein expression is detected in secretory epithelial cells as well as in the luminal space of the alveoli.
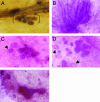
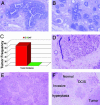
Fig. 5.
Hyperplastic and tumorigenic outgrowths derived from reduction mammoplasty epithelium. Hematoxylin/eosin-stained sections of hyperplasias that developed from normal organoids in the absence of normal admixed fibroblasts (A and B). (C) Frequency of tumor formation when reduction mammoplasty organoids were introduced directly into humanized stroma overexpressing TGF-β or HGF (O) or when the organoids were coinjected with normal primary human breast fibroblasts (O + F). Histology of the tumors arising in glands humanized with growth factor expressing stroma: stroma that overexpresses either HGF (D) or TGF-β1 (E). Histology of outgrowths of various presumed stages of human breast cancer detected in the same field. Normal ductal tissue (normal), benign hyperplastic ducts (hyperplasia), in situ ductal cancer (DCIS), and invasive carcinoma (invasive) (F).
Comment in
- Stromal fibroblasts influence human mammary epithelial cell morphogenesis.
Medina D. Medina D. Proc Natl Acad Sci U S A. 2004 Apr 6;101(14):4723-4. doi: 10.1073/pnas.0401376101. Epub 2004 Mar 29. Proc Natl Acad Sci U S A. 2004. PMID: 15051868 Free PMC article. No abstract available.
Similar articles
- Fibroblasts direct differentiation of human breast epithelial progenitors.
Morsing M, Kim J, Villadsen R, Goldhammer N, Jafari A, Kassem M, Petersen OW, Rønnov-Jessen L. Morsing M, et al. Breast Cancer Res. 2020 Sep 29;22(1):102. doi: 10.1186/s13058-020-01344-0. Breast Cancer Res. 2020. PMID: 32993755 Free PMC article. - Reconstruction of human mammary tissues in a mouse model.
Proia DA, Kuperwasser C. Proia DA, et al. Nat Protoc. 2006;1(1):206-14. doi: 10.1038/nprot.2006.31. Nat Protoc. 2006. PMID: 17406234 - Epithelial-stromal interactions in the mouse and human mammary gland in vivo.
Parmar H, Cunha GR. Parmar H, et al. Endocr Relat Cancer. 2004 Sep;11(3):437-58. doi: 10.1677/erc.1.00659. Endocr Relat Cancer. 2004. PMID: 15369447 Review. - Mammary gland stem cells: current status and future challenges.
Fridriksdottir AJ, Petersen OW, Rønnov-Jessen L. Fridriksdottir AJ, et al. Int J Dev Biol. 2011;55(7-9):719-29. doi: 10.1387/ijdb.113373af. Int J Dev Biol. 2011. PMID: 22161829 Review.
Cited by
- Reprogramming of Normal Fibroblasts into Cancer-Associated Fibroblasts by miRNAs-Mediated CCL2/VEGFA Signaling.
Shen H, Yu X, Yang F, Zhang Z, Shen J, Sun J, Choksi S, Jitkaew S, Shu Y. Shen H, et al. PLoS Genet. 2016 Aug 19;12(8):e1006244. doi: 10.1371/journal.pgen.1006244. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27541266 Free PMC article. - Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma.
Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts LR, Sirica AE, Gores GJ. Mertens JC, et al. Cancer Res. 2013 Jan 15;73(2):897-907. doi: 10.1158/0008-5472.CAN-12-2130. Epub 2012 Dec 5. Cancer Res. 2013. PMID: 23221385 Free PMC article. - The Role of Stroma in Tumor Development.
Werb Z, Lu P. Werb Z, et al. Cancer J. 2015 Jul-Aug;21(4):250-3. doi: 10.1097/PPO.0000000000000127. Cancer J. 2015. PMID: 26222075 Free PMC article. Review. - The evolution of the cancer niche during multistage carcinogenesis.
Barcellos-Hoff MH, Lyden D, Wang TC. Barcellos-Hoff MH, et al. Nat Rev Cancer. 2013 Jul;13(7):511-8. doi: 10.1038/nrc3536. Epub 2013 Jun 13. Nat Rev Cancer. 2013. PMID: 23760023 Review. - Single unpurified breast tumor-initiating cells from multiple mouse models efficiently elicit tumors in immune-competent hosts.
Kurpios NA, Girgis-Gabardo A, Hallett RM, Rogers S, Gludish DW, Kockeritz L, Woodgett J, Cardiff R, Hassell JA. Kurpios NA, et al. PLoS One. 2013;8(3):e58151. doi: 10.1371/journal.pone.0058151. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555570 Free PMC article.
References
- DeOme, K. B., Faulkin, L. J., Jr., Bern, H. & Blair, P. B. (1959) Cancer Res. 19, 515-525. - PubMed
- Outzen, H. C. & Custer, R. P. (1975) J. Natl. Cancer Inst. 55, 1461-1466. - PubMed
- Sheffield, L. G. & Welsch, C. W. (1988) Int. J. Cancer 41, 713-719. - PubMed
- McManus, M. J. & Welsch, C. W. (1981) Cancer Res. 41, 3300-3305. - PubMed
- Yang, J., Liu, A., Dougherty, C., Chen, X., Guzman, R. & Nandi, S. (2000) Oncol. Rep. 7, 17-21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources