Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? - PubMed (original) (raw)
Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts?
Dorothy P Schafer et al. J Neurosci. 2004.
Abstract
Paranodal axoglial junctions in myelinated nerve fibers are essential for efficient action potential conduction and ion channel clustering. We show here that, in the mature CNS, a fraction of the oligodendroglial 155 kDa isoform of neurofascin (NF-155), a major constituent of paranodal junctions, has key biochemical characteristics of a lipid raft-associated protein. However, despite its robust expression, NF-155 is detergent soluble before paranodes form and in purified oligodendrocyte cell cultures. Only during its progressive localization to paranodes is NF-155 (1) associated with detergent-insoluble complexes that float at increasingly lower densities of sucrose and (2) retained in situ after detergent treatment. Finally, mutant animals with disrupted paranodal junctions, including those lacking specific myelin lipids, have significantly reduced levels of raft-associated NF-155. Together, these results suggest that trans interactions between oligodendroglial NF-155 and axonal ligands result in cross-linking, stabilization, and formation of paranodal lipid raft assemblies.
Figures
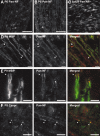
Figure 1.
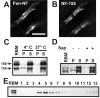
A subpopulation of NF-155 has key biochemical characteristics of a lipid raft-associated protein. A, B, Rat optic nerve sections double labeled for pan-NF and NF-155. C–E, Analysis of rat brain membranes (RBM). C, Immunoblot showing the fractionation of NF isoforms into the 1% TX-100 detergent-insoluble fraction (or pellet) or soluble fraction at either 4°C or 37°C. Each temperature condition shows the equivalent of 10 μg of protein split between soluble and pellet fractions. The RBM lane was loaded with 10 μg of protein. All immunoblots (C–F) were performed using pan-NF antibodies. D, Previous cholesterol extraction from brain membrane homogenate by 0.2% saponin at 4°C promotes the solubility of NF-155 in 1% TX-100 at 4°C. S, 1% TX-100-soluble fraction. P, 1% TX-100-insoluble pellet. E, Sucrose density gradient analysis of the detergent-insoluble fraction immunoblotted for NF isoforms. Scale bars, 5 μm.
Figure 7.
Raft-associated NF-155 is reduced in rodents with disrupted paranodes. A, B, Nodes of Ranvier from sciatic nerves (A) and optic nerves (B) of WT and CST-null (CST) and CGT-null (CGT) mice double labeled for NF-155 (green) and Na+ channels (red). (Note that the axons run horizontally in A and vertically in B.) Rabbit polyclonal anti-NF-155 was used in all experiments shown (A–E). C, Immunoblots of sucrose gradients using detergent-insoluble pellets from P30 WT and CGT-null mouse brains assayed for NF-155, Caspr, contactin, and GM1. D, A comparison of the NF-155 protein levels in P30 WT and CGT-null mouse brains. Each lane was loaded with 40 μg of brain membrane proteins. E, Mouse oligodendrocyte cultures from WT and CGT-null mice. NF-155 and CNP immunoreactivity from cultures from four mouse pups, two of each genotype are shown. These experiments were repeated in two independent litters of animals with identical results. F, Immunoblot of brain membranes from WT and md rats showing (1% TX-100 at 4°C) soluble (S) and insoluble (P) fractions. Immunoblots were performed using anti-pan-NF antibodies (arrow indicates NF-155). Scale bars, 5 μm.
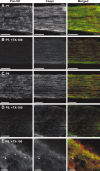
Figure 2.
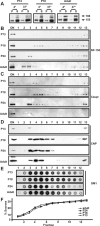
The density and detergent insolubility of NF-155 are developmentally regulated. A, Detergent extraction of NF-155 from P12, P18, and adult rat optic nerve membrane homogenates. Each temperature condition shows the equivalent of 10 μg of protein split between soluble (S) and pellet (P) fractions. Immunoblotting was performed using the pan-NF antibody_. B–E_, Detergent-insoluble pellets from P13, P18, P24, and adult rat optic nerve (ON) homogenates were floated on sucrose gradients. Equal volume fractions were collected from the gradients and immunoblotted using pan-NF (B), Caspr(C), or anti-CNP (D). Equal volumes from each fraction were loaded on the gels. The ganglioside GM1 was assayed by dot blot using peroxidase-conjugated cholera toxin (E). One microliter of each fraction was loaded. Pellets from the gradients (fraction 13) are not shown. F, The percentage of sucrose was measured for each fraction used in the experiments shown in B–E.
Figure 3.
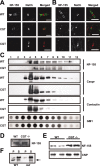
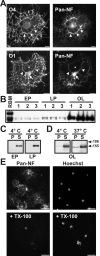
NF-155 is expressed at high levels in cultured oligodendrocytes and is soluble in 1% TX-100. A, Immunolabeling of purified oligodendrocytes using pan-NF and O4 or O1 antibodies. Oligodendrocytes expressed NF-155 in the cell body (arrow) and major processes (arrowheads), but NF-155 was excluded from the membrane sheets (asterisk). B, Homogenates from three separate populations of early progenitors (EP), late progenitors (LP), and oligodendrocytes (OL) immunoblotted using pan-NF; NF-186 was not detected. C, D, 1% TX-100 solubilization of oligodendrocytes and its progenitors at either 4°C or 37°C. E, Cultured oligodendrocytes stained for NF-155 and Hoechst with and without previous detergent extraction of live cells in 1% TX-100 at 4°C. Scale bars, 20 μm.
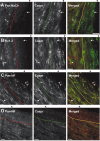
Figure 4.
NF-155 expression and localization during myelination. A, B, pan-NF immunolabeling of P6 and P8 rat optic nerve. Pronounced NF-155 staining can be seen in the cell bodies of oligodendrocytes (arrowheads). C, Adult optic nerve labeled with pan-NF. Immunostaining is restricted to nodes and paranodes (arrowhead). D, Double labeling of rat optic nerves at P8 with antibodies against MBP (green) and pan-NF (red). NF-155 is localized at the ends of MBP-labeled oligodendrocyte processes (arrowheads). An oligodendrocyte cell body is identified by the asterisk. E, Double labeling of rat optic nerves at P9 with antibodies against MBP (green) and pan-NF (red). NF-155 is nested within MBP staining and is localized at the ends of the MBP-labeled oligodendrocyte processes (arrowheads). F, Double labeling of rat optic nerve at P8 using antibodies against Caspr (green) and pan-NF (red). Staining for NF-155 and Caspr is colocalized at forming paranodes (arrowheads). Scale bars: A, B, 100 μm; C, D, 10 μm; E, F, 5 μm.
Figure 5.
NF-155 is readily extracted from optic nerves before paranode formation. Sections were double labeled using pan-NF (red) and antibodies against Caspr (green). At early stages of myelination, TX-100 penetrated completely through the nerve because of the fact that the tissue is much less dense than in adults and the nerves are much smaller. A, P7 optic nerve. B, P7 optic nerve after detergent treatment in 1% TX-100 at 4°C. C, P9 optic nerve. D, P9 optic nerve after detergent treatment in 1% TX-100 at 4°C. E, Detergent treatment of P9 rat optic nerve showed that NF-155 and Caspr immunoreactivity was retained at forming paranodes (arrowheads). Scale bars: A–D, 100 μm; E,10 μm.
Figure 6.
Paranodal NF-155 and Caspr are resistant to detergent extraction from whole optic nerves. A, B, Detergent-treated rat optic nerves immunolabeled for voltage-dependent Na+ channels (red, Pan NaCh) or Kv1.2 K+ channels (red) and Caspr (green). Black arrowheads above the panels indicate the edge of the nerve. The line through each panel approximates the depth to which the detergent penetrated and the color of the fluorophore used. In regions in which detergent penetrated Na channels and Kv1.2 were efficiently extracted from nodes (A, arrow) and juxtaparanodes (B, arrow), respectively. Both were retained in regions in which detergent did not penetrate (A, B, arrowhead). C, Double labeling of detergent-treated optic nerves using antibodies against Caspr (green) and pan-NF (red). Paranodal NF-155 and Caspr are retained after detergent treatment (double arrowheads), but nodal NF-186 is extracted. In regions in which detergent did not penetrate, nodal NF-186 is retained (arrow). D, Detergent treatment at 37°C, followed by double labeling for neurofascin (red) and Caspr (green). Scale bars: A, B,10 μm; C, 5 μm; D, 20 μm.
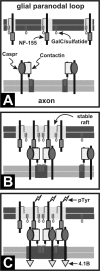
Figure 8.
Model for lipid raft-dependent paranode formation. A, Caspr, contactin, and NF-155 are initially unclustered, not present in rafts, and readily solubilized by 1% TX-100. B, During binding to its axonal ligand in trans, NF-155 and its associated lipid environment are stabilized and become detergent insoluble. Additional NF-155 is recruited through thermodynamically favorable inclusion into the lipid environment surrounding the trans stabilized NF-155. C, Binding of additional Caspr/contactin (or another axonal ligand) to NF-155 then results in a stable paranodal structure with distinct lipid environments in both the axon and oligodendrocyte. This paranodal structure may then be involved in cytoskeletal interactions through protein 4.1B and signaling through phosphorylation of NF-155 (pTyr). GalC, Galactocerebroside.
Similar articles
- Proteomic analysis of optic nerve lipid rafts reveals new paranodal proteins.
Ogawa Y, Rasband MN. Ogawa Y, et al. J Neurosci Res. 2009 Nov 15;87(15):3502-10. doi: 10.1002/jnr.21984. J Neurosci Res. 2009. PMID: 19156860 Free PMC article. - Accumulation of Neurofascin at Nodes of Ranvier Is Regulated by a Paranodal Switch.
Zhang Y, Yuen S, Peles E, Salzer JL. Zhang Y, et al. J Neurosci. 2020 Jul 22;40(30):5709-5723. doi: 10.1523/JNEUROSCI.0830-19.2020. Epub 2020 Jun 17. J Neurosci. 2020. PMID: 32554548 Free PMC article. - The raft-associated protein MAL is required for maintenance of proper axon--glia interactions in the central nervous system.
Schaeren-Wiemers N, Bonnet A, Erb M, Erne B, Bartsch U, Kern F, Mantei N, Sherman D, Suter U. Schaeren-Wiemers N, et al. J Cell Biol. 2004 Aug 30;166(5):731-42. doi: 10.1083/jcb.200406092. J Cell Biol. 2004. PMID: 15337780 Free PMC article. - GPI-anchored proteins at the node of Ranvier.
Labasque M, Faivre-Sarrailh C. Labasque M, et al. FEBS Lett. 2010 May 3;584(9):1787-92. doi: 10.1016/j.febslet.2009.08.025. Epub 2009 Aug 22. FEBS Lett. 2010. PMID: 19703450 Review. - Glial regulation of the axonal membrane at nodes of Ranvier.
Schafer DP, Rasband MN. Schafer DP, et al. Curr Opin Neurobiol. 2006 Oct;16(5):508-14. doi: 10.1016/j.conb.2006.08.003. Epub 2006 Sep 1. Curr Opin Neurobiol. 2006. PMID: 16945520 Review.
Cited by
- The study of microtubule dynamics and stability at the postsynaptic density in a rat pilocarpine model of temporal lobe epilepsy.
Wu X, Zhou Y, Huang Z, Cai M, Shu Y, Zeng C, Feng L, Xiao B, Zhan Q. Wu X, et al. Ann Transl Med. 2020 Jul;8(14):863. doi: 10.21037/atm-19-4636. Ann Transl Med. 2020. PMID: 32793707 Free PMC article. - Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo.
Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. Brackenbury WJ, et al. J Neurosci. 2008 Mar 19;28(12):3246-56. doi: 10.1523/JNEUROSCI.5446-07.2008. J Neurosci. 2008. PMID: 18354028 Free PMC article. - Sphingolipids: membrane microdomains in brain development, function and neurological diseases.
Olsen ASB, Færgeman NJ. Olsen ASB, et al. Open Biol. 2017 May;7(5):170069. doi: 10.1098/rsob.170069. Open Biol. 2017. PMID: 28566300 Free PMC article. Review. - Oligodendrocyte development and myelin biogenesis: parsing out the roles of glycosphingolipids.
Jackman N, Ishii A, Bansal R. Jackman N, et al. Physiology (Bethesda). 2009 Oct;24:290-7. doi: 10.1152/physiol.00016.2009. Physiology (Bethesda). 2009. PMID: 19815855 Free PMC article. Review. - Dysfunction of nodes of Ranvier: a mechanism for anti-ganglioside antibody-mediated neuropathies.
Susuki K, Yuki N, Schafer DP, Hirata K, Zhang G, Funakoshi K, Rasband MN. Susuki K, et al. Exp Neurol. 2012 Jan;233(1):534-42. doi: 10.1016/j.expneurol.2011.11.039. Epub 2011 Dec 8. Exp Neurol. 2012. PMID: 22178332 Free PMC article.
References
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE (1996) Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci 7: 263–275. - PubMed
- Baron W, Decker L, Colognato H, ffrench-Constant C (2003) Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol 13: 151–155. - PubMed
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St. Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ (2001) Axonglia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30: 369–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038878/NS/NINDS NIH HHS/United States
- NS44916/NS/NINDS NIH HHS/United States
- NS10861/NS/NINDS NIH HHS/United States
- F31 NS010861/NS/NINDS NIH HHS/United States
- R01 NS010861/NS/NINDS NIH HHS/United States
- NS41078/NS/NINDS NIH HHS/United States
- R01 NS041078/NS/NINDS NIH HHS/United States
- R37 NS044916/NS/NINDS NIH HHS/United States
- NS38878/NS/NINDS NIH HHS/United States
- R37 NS038878/NS/NINDS NIH HHS/United States
- R01 NS044916/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous