14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1) - PubMed (original) (raw)
Comparative Study
. 2004 May 5;23(9):1934-42.
doi: 10.1038/sj.emboj.7600198. Epub 2004 Apr 1.
Affiliations
- PMID: 15057270
- PMCID: PMC404318
- DOI: 10.1038/sj.emboj.7600198
Comparative Study
14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1)
Toshihiro Sekimoto et al. EMBO J. 2004.
Abstract
p27(Kip1) (p27), a CDK inhibitor, migrates into the nucleus, where it controls cyclin-CDK complex activity for proper cell cycle progression. We report here that the classical bipartite-type basic amino-acid cluster and the two downstream amino acids of the C-terminal region of p27 function as a nuclear localization signal (NLS) for its full nuclear import activity. Importin alpha3 and alpha5, but not alpha1, transported p27 into the nucleus in conjunction with importin beta, as evidenced by an in vitro transport assay. It is known that Akt phosphorylates Thr 157 of p27 and this reduces the nuclear import activity of p27. Using a pull-down experiment, 14-3-3 was identified as the Thr157-phosphorylated p27NLS-binding protein. Although importin alpha5 bound to Thr157-phosphorylated p27NLS, 14-3-3 competed with importin alpha5 for binding to it. Thus, 14-3-3 sequestered phosphorylated p27NLS from importin alpha binding, resulting in cytoplasmic localization of NLS-phosphorylated p27. These findings indicate that 14-3-3 suppresses importin alpha/beta-dependent nuclear localization of Thr157-phosphorylated p27, suggesting implications for cell cycle disorder in Akt-activated cancer cells.
Figures
Figure 1
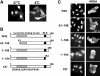
C-terminal region participates in the active nuclear import of p27. (A) Purified recombinant Flag-p27 was microinjected into the HeLa cytoplasm. After 30 min at 37 or 4°C, cells were fixed and the subcellular localization of Flag-p27 was detected by indirect immunofluorescence using anti-Flag antibody. (B) Flag-p27 mutants used in this experiment. (C) Nuclear import activity of Flag-p27 mutants. Purified Flag-p27 mutants were microinjected with or without WGA into the HeLa cytoplasm.
Figure 2
NLS of p27 is bipartite type. (A) Sequences inserted into GST–GFP fusion proteins are listed. The basic amino-acid clusters are underlined, and substituted or inserted amino acids are in bold letters. (B; a) GST–GFP alone (−) or NLS of SV40 (SV40 NLS)-inserted GST–GFP was microinjected into the HeLa cytoplasm. (b) Nuclear import activity of p27NLS-inserted GST–GFP fusion proteins. Proteins were microinjected into the HeLa cytoplasm. (C) Full-length Flag-p27 (wild type, K165A/R166A or A167G/N168G mutant) was microinjected into the HeLa cytoplasm. The relative nuclear to cytoplasmic ratio (average values for 20 injected cells) was represented as percentage.
Figure 3
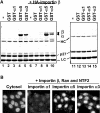
Importin α3- and α5-dependent nuclear import of p27. (A) Solution-binding assay. Flag-p27 was incubated with GST or GST-importin α's with (lanes 1–5) or without (lanes 6–10) HA-importin β in the presence of anti-Flag agarose. The same experiment was carried out using Flag-p27 (K165A/R166A) without importin β (lanes 11–15). The bound proteins were eluted and analyzed by SDS–PAGE. GST-importin α1, α3 and α5 are indicated as GST-α1, -α3 and -α5. HC: heavy chain and LC: light chain. (B) In vitro transport assay. Flag-p27 was applied to the in vitro reconstituted transport assay system supplemented with Ehrlich cytosol, or importin α/β with Ran and NTF2.
Figure 4
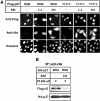
Phosphorylation by Akt increased cytoplasmic p27. (A) HeLa cells were transfected with pcDNA-Flag-p27 (wild or T157A) together with empty vector (−) or pcDNA-Akt (CA or DN) at 1:9 ratio for 24 h. DNA was stained by Hoechst dye. CA, constitutively active form. DN, dominant-negative form. (B) Phosphorylation of p27 by Akt reduced importin α5 binding. 293T cells were transfected with pcDNA-HA-p27 (wild) and pcDNA-Flag-importin α5 (Flag-α5) together with empty vector (−) or pcDNA-Akt (CA). HA-p27 was immunoprecipitated (IP) and co-precipitated proteins were analyzed by Western blotting using anti-Flag or anti-HA antibody.
Figure 5
Phosphorylation at Thr157 reduces the nuclear import activity of p27NLS. (A) Conjugates used in this study. BSA was labeled with biotin, and then conjugated with nonphosphorylated or Thr157-phosphorylated p27NLS peptides. (B) Nuclear import activity of conjugates. Conjugates were microinjected into the HeLa cytoplasm with RITC-BSA. (C) Solution-binding assay. Conjugates were incubated with Flag-importin α1 (lanes 2–4) or α5 (lanes 6–8) in the presence of avidin agarose. The bound proteins were eluted and analyzed by SDS–PAGE. Minus (−) indicates incubation of importin α's with avidin agarose only. (D) In vitro transport assay. Conjugates were applied to the in vitro reconstituted transport assay system using Ehrlich cytosol, or importin α5/β with Ran and NTF2.
Figure 6
14-3-3 associates with Thr157-phosphorylated p27NLS. (A) Identification of Thr157-phosphorylated p27NLS-binding protein. HeLa cytosol was incubated with bBSA-NLS or bBSA-pNLS immobilized to avidin agarose. Bound proteins were separated by SDS–PAGE. Arrows indicate pNLS-specific binding proteins (left panel). The same samples were subjected to Western blotting and bound protein was detected by anti-14-3-3 antibody (right panel). (B) 14-3-3 associates with Thr157-phosphorylated p27 in vivo. 293T cells were transfected with pcDNA-Flag-p27 (wild, T157A or 1–188) together with empty vector (−; lanes 1,4 and 7) or pcDNA-Akt (CA or DN; lanes 2, 3, 5, 6, 8 and 9) at 1:9 ratio. Flag-p27 was immunoprecipitated (IP), and co-precipitated proteins were analyzed by Western blotting using anti-Flag or anti-14-3-3 antibody. Asterisk (*) indicates the light chain of IgG.
Figure 7
14-3-3 masks phosphorylated-p27NLS from importin α binding. (A) Specific 14-3-3 isoforms bind to phosphorylated p27NLS. Recombinant HA-14-3-3 isoforms (β, ɛ, γ, σ, θ and ζ) were incubated with bBSA-NLS (lanes 1, 3, 5, 7, 9 and 11) or bBSA-pNLS (lanes 2, 4, 6, 8, 10 and 12). The bound proteins were eluted and analyzed by SDS–PAGE. (B) 14-3-3 competes with importin α for binding to Thr157-phosphorylated p27NLS. Importin α5 was incubated with bBSA-NLS (lanes 2–5) or bBSA-pNLS (lanes 7–10) in the presence of increasing amounts of HA-14-3-3γ (lanes 3–5 and 8–10). The amounts of added HA-14-3-3 are 2 (lanes 3 and 8), 6 (lanes 4 and 9) and 16 (lanes 5 and 10) molar excess of importin α5. (C) 14-3-3 inhibits the nuclear import of Thr157-phosphorylated p27NLS in vitro. Conjugates were subjected to the in vitro reconstituted transport assay using importin α5/β with Ran and NTF2 in the presence of increasing amounts of HA-14-3-3γ. The amounts of added HA-14-3-3 are 8 and 24 molar excess of importin α5.
Similar articles
- Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer.
Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, Fusco A, Santoro M. Viglietto G, et al. Nat Med. 2002 Oct;8(10):1136-44. doi: 10.1038/nm762. Epub 2002 Sep 16. Nat Med. 2002. PMID: 12244303 - PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. Liang J, et al. Nat Med. 2002 Oct;8(10):1153-60. doi: 10.1038/nm761. Epub 2002 Sep 16. Nat Med. 2002. PMID: 12244302 - p27KIP1 phosphorylation by PKB/Akt leads to poor breast cancer prognosis.
Clarke RB. Clarke RB. Breast Cancer Res. 2003;5(3):162-3. doi: 10.1186/bcr596. Epub 2003 Mar 31. Breast Cancer Res. 2003. PMID: 12793899 Free PMC article. Review. No abstract available. - Keeping p27(Kip1) in the cytoplasm: a second front in cancer's war on p27.
Reed SI. Reed SI. Cell Cycle. 2002 Nov-Dec;1(6):389-90. doi: 10.4161/cc.1.6.261. Cell Cycle. 2002. PMID: 12548010 Review. No abstract available.
Cited by
- Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin.
Grunewald W, Vanholme B, Pauwels L, Plovie E, Inzé D, Gheysen G, Goossens A. Grunewald W, et al. EMBO Rep. 2009 Aug;10(8):923-8. doi: 10.1038/embor.2009.103. Epub 2009 Jul 3. EMBO Rep. 2009. PMID: 19575013 Free PMC article. - 14-3-3ζ orchestrates mammary tumor onset and progression via miR-221-mediated cell proliferation.
Rehman SK, Li SH, Wyszomierski SL, Wang Q, Li P, Sahin O, Xiao Y, Zhang S, Xiong Y, Yang J, Wang H, Guo H, Zhang JD, Medina D, Muller WJ, Yu D. Rehman SK, et al. Cancer Res. 2014 Jan 1;74(1):363-373. doi: 10.1158/0008-5472.CAN-13-2016. Epub 2013 Nov 6. Cancer Res. 2014. PMID: 24197133 Free PMC article. - Importin alpha protein acts as a negative regulator for Snail protein nuclear import.
Sekimoto T, Miyamoto Y, Arai S, Yoneda Y. Sekimoto T, et al. J Biol Chem. 2011 Apr 29;286(17):15126-31. doi: 10.1074/jbc.M110.213579. Epub 2011 Mar 17. J Biol Chem. 2011. PMID: 21454664 Free PMC article. - The B56γ3 regulatory subunit-containing protein phosphatase 2A outcompetes Akt to regulate p27KIP1 subcellular localization by selectively dephosphorylating phospho-Thr157 of p27KIP1.
Lai TY, Yen CJ, Tsai HW, Yang YS, Hong WF, Chiang CW. Lai TY, et al. Oncotarget. 2016 Jan 26;7(4):4542-58. doi: 10.18632/oncotarget.6609. Oncotarget. 2016. PMID: 26684356 Free PMC article. - Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition.
Katayama K, Fujita N, Tsuruo T. Katayama K, et al. Mol Cell Biol. 2005 Jul;25(13):5725-37. doi: 10.1128/MCB.25.13.5725-5737.2005. Mol Cell Biol. 2005. PMID: 15964826 Free PMC article.
References
- Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone MV, Chiappetta G, Vento MT, Spiezia S, Fusco A, Viglietto G (1999) Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Invest 104: 865–874 - PMC - PubMed
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 - PubMed
- Carrano AC, Eytan E, Hershko A, Pagano M (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1: 193–199 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous