Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(-/-)/Tcf1(-/-) embryos - PubMed (original) (raw)
Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(-/-)/Tcf1(-/-) embryos
Alex Gregorieff et al. EMBO J. 2004.
Abstract
Wnt signalling plays a critical role in both initiating and patterning of the anterior-posterior axis during development. Wnts exert their biological effects, in part, by activating specific target genes through members of the TCF/LEF family of transcription factors. To gain new insight into the role of T-cell factors (or Tcf's) during development, we analysed Tcf4 and Tcf1 compound null embryos. These mutants showed severe caudal truncations, as well as duplications of the neural tube. Unlike other mutations affecting Wnt signalling, paraxial mesoderm formation was not impaired and early caudal markers, such as T, were unaffected. Analysis of endodermal markers uncovered early and specific defects in hindgut expansion, and later an anterior transformation of the gastro-intestinal tract. Our results reveal a novel role for Wnt signalling in early gut morphogenesis and suggest that specific Wnt-driven patterning events are determined by the unique tissue distribution of Tcf/Lef family members.
Figures
Figure 1
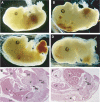
Tcf4_−/−/Tcf1_−/− embryos show severe caudal truncations. Lateral views of E14.5 embryos. (A) Tcf4+/−_/Tcf1_−/− and (B–D) Tcf4_−/−/Tcf1_−/− littermates. Panel B shows vestigial hindlimbs and the absence of a tail in mutant embryos. In more severe cases as depicted in panels C and D, internal organs such as the gut and liver are exposed. In several instances, the neural tube branched out in mutant embryos as shown in (D, F) (see arrow). Panels E and F represent haematoxylin and eosin stainings of sections of embryos depicted in (A, B), respectively. Various structures are identified as follows: (H) heart, (Lu) lungs, (L) liver, (K) kidneys, (U) urogenital sinus, (MD) mesonephric duct, (GT) genital tubercle, (SI) small intestine, (HG) hindgut and (N) neural tube.
Figure 2
Duplications of the neural tube in Tcf4_−/−/Tcf1_−/− embryos. Whole-mount in situ hybridizations of E10.5 littermates using a Wnt1 probe as a marker for neural tube. (A) Tcf4+/−_/Tcf1_−/− embryo; (B) right side, (C) left side and (D) close-up views of a representative Tcf4_−/−/Tcf1_−/− embryo. Lateral and close-up views reveal that the neural tube appears to split at the caudal end of mutant embryos. In some regions (see the arrow in (D) and the corresponding section in (E)), complete duplications of the neural tube are observed. Note that Wnt1 expression appears normal in the anterior half of Tcf4_−/−/Tcf1_−/− embryos.
Figure 3
Paraxial mesoderm is intact in Tcf4_−/−/Tcf1_−/− embryos. Whole-mount in situ hybridizations of E10.5 littermates using markers for paraxial mesoderm. (A, C, E) Tcf4+/−_/Tcf1_−/− embryos and (B, D, F) Tcf4_−/−/Tcf1_−/− embryos. Panels A and B show stainings for Paraxis, panels C and D show Pax1 stainings. Panels E and F show expression of Myf5. All three somitic markers confirm the presence of paraxial mesoderm throughout the axis of Tcf4_−/−/Tcf1_−/− mutants.
Figure 4
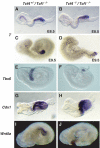
Expression of early posterior markers is unaffected in Tcf4_−/−/Tcf1_−/− embryos. Whole-mount in situ hybridizations of E8.5–9.5 littermates using various posterior markers (see above). (A, C, E, G, I) Tcf4+/−_/Tcf1_−/− embryos and (B, D, F, H, J) Tcf4_−/−/Tcf1_−/− embryos. Panels (A, B) and (C, D) represent E8.5 and E9.5 embryos, respectively, and show that initiation and maintenance of T expression is unchanged in Tcf4_−/−/Tcf1_−/− embryos.
Figure 5
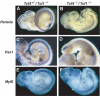
Comparison of Tcf4, Tcf1 and Lef expression. In situ hybridizations on sections of E8.5 and E10.5 wild-type embryos were performed using specific probes recognizing Tcf4 (A, D), Tcf1 (B, E) and Lef (C, F). Top panels A–C depict sections through the primitive streak region of E8.5 embryos, while bottom panels D–E show sections through the tail bud of E10.5 embryos. Various structures are identified as follows: (PE) primitive ectoderm, (NT) neural tube, (PSM) presomitic mesoderm and (HG) hindgut. Tcf4 was specifically expressed in the hindgut at E8.5 and E10.5 in the neural tube and hindgut. Tcf1 was found in all three germ layers at both E8.5 and E10.5. Lef was detected in the presomitic mesoderm in both stages examined and was absent from the primitive gut.
Figure 6
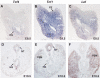
Impaired expansion of caudal endoderm in Tcf4_−/−/Tcf1_−/−embryos. Whole-mount in situ hybridizations of E8.5–9.5 littermates using endodermal markers and histology of E9.5 normal and mutant embryos. (A, D, G, J, M) Wild-type embryos, (B, E, H, K, N) Tcf4_−/−/Tcf1_−/−embryos and (C, F, I, L, O) Lef_−/−/Tcf1_−/− embryos. The arrows in (B) and (E) point to the loss of Sox17 and Foxa1 staining in the caudal endoderm of Tcf4_−/−/Tcf1_−/−, while staining is normal in Lef_−/−/Tcf1_−/− embryos. Arrows in (G) and (I) point to Shh expression in the hindgut of wild-type and Lef_−/−/Tcf1_−/− embryos. The notochord in Tcf4_−/−/Tcf1_−/−embryos appears exposed due to the absence of an underlying gut tube (arrowhead in (H)). Panels J–L and M–O represent haematoxylin and eosin stainings of sections through the midgut region and hindgut, respectively. Various structures are identified as follows: (S) somites, (NT) neural tube, (MG) midgut and (HG) hindgut. Note that in panel K Tcf4_−/−/Tcf1_−/− embryos maintain somites but the primitive gut tube has not closed. Similar sections in Lef_−/−/Tcf1_−/− embryos show a properly formed gut tube and the absence of somites (panel L). The caudal sections (M–O) also reveal the presence of ectopic neural tissue in both Tcf4_−/−/Tcf1_−/− and Lef_−/−/Tcf1_−/− embryos.
Figure 7
Anteriorization of the gastro-intestinal tract in Tcf4_−/−/Tcf1_−/− embryos. (A) Whole-mount in situ hybridizations of dissected anterior gastro-intestinal tracts of E14.5 Tcf4+/−_/Tcf1_−/− (left) and Tcf4_−/−/Tcf1_−/− (right) embryos using a stomach marker, Sox2. The gastro-intestinal tract of the normal embryo was severed to remove the intestinal tube. The truncated gastro-intestinal tract of Tcf4_−/−/Tcf1_−/− embryos is shown in its entirety. As shown by the arrowheads, Sox2 expression is confined to the stomach in normal littermates, while ectopic expression in the duodenum is apparent in mutants. (B) In situ hybridizations on consecutive sections (i–iv) of a single E13.5 Tcf4+/−_/Tcf1_−/− gastro-duodenal preparation. The top and bottom rows of panels were stained for Sox2 (stomach) and Cdx2 (intestine), respectively. The top and bottom sections i–iv represent equivalent regions and should be compared with each other. The arrowheads indicate regions of positive staining for both probes. (C) In situ hybridizations on consecutive sections (i–vi) of a single E13.5 Tcf4_−/−/Tcf1_−/− gastro-duodenal preparation. The top and bottom sets of panels were stained for Sox2 and Cdx2, respectively. Sections i–vi represent equivalent regions and should be compared with each other. Various structures are identified as follows: (S) stomach, (D) duodenum. The arrowheads in panels stained for Cdx2 represent regions of the duodenum that are devoid of Cdx2 expression but show high expression of Sox2. Note how the duodenum in mutants appears dilated compared to normal littermates (B). In addition, Cdx2 transcripts are confined to a small portion of the duodenum, and the remaining tissue expresses Sox2. Altogether, these data provide evidence for the occurrence of an anterior transformation in the gastro-intestinal tract of Tcf4_−/−/Tcf1_−/− embryos.
Similar articles
- Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse.
Engert S, Burtscher I, Liao WP, Dulev S, Schotta G, Lickert H. Engert S, et al. Development. 2013 Aug;140(15):3128-38. doi: 10.1242/dev.088765. Epub 2013 Jul 3. Development. 2013. PMID: 23824574 - Gene expression analysis of canonical Wnt pathway transcriptional regulators during early morphogenesis of the facial region in the mouse embryo.
Vendrell V, Summerhurst K, Sharpe J, Davidson D, Murphy P. Vendrell V, et al. Gene Expr Patterns. 2009 Jun;9(5):296-305. doi: 10.1016/j.gep.2009.03.001. Epub 2009 Mar 19. Gene Expr Patterns. 2009. PMID: 19303461 - Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development.
Liu F, van den Broek O, Destrée O, Hoppler S. Liu F, et al. Development. 2005 Dec;132(24):5375-85. doi: 10.1242/dev.02152. Epub 2005 Nov 16. Development. 2005. PMID: 16291789 - Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression.
Galceran J, Hsu SC, Grosschedl R. Galceran J, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8668-73. doi: 10.1073/pnas.151258098. Epub 2001 Jul 10. Proc Natl Acad Sci U S A. 2001. PMID: 11447280 Free PMC article. - Recent advances in the molecular and genetic understanding of congenital gastrointestinal malformations.
Dauvé V, McLin VA. Dauvé V, et al. J Pediatr Gastroenterol Nutr. 2013 Jul;57(1):4-13. doi: 10.1097/MPG.0b013e3182922b49. J Pediatr Gastroenterol Nutr. 2013. PMID: 23539045 Review.
Cited by
- Conservation of _cis_-Regulatory Syntax Underlying Deuterostome Gastrulation.
Buono L, Annona G, Magri MS, Negueruela S, Sepe RM, Caccavale F, Maeso I, Arnone MI, D'Aniello S. Buono L, et al. Cells. 2024 Jun 28;13(13):1121. doi: 10.3390/cells13131121. Cells. 2024. PMID: 38994973 Free PMC article. - Spatiotemporal map of key signaling factors during early penis development.
Tarulli GA, Cripps SM, Pask AJ, Renfree MB. Tarulli GA, et al. Dev Dyn. 2022 Apr;251(4):609-624. doi: 10.1002/dvdy.433. Epub 2021 Nov 6. Dev Dyn. 2022. PMID: 34697862 Free PMC article. Review. - Negative regulation of IL-8 in human astrocytes depends on β-catenin while positive regulation is mediated by TCFs/LEF/ATF2 interaction.
Robinson KF, Narasipura SD, Wallace J, Ritz EM, Al-Harthi L. Robinson KF, et al. Cytokine. 2020 Dec;136:155252. doi: 10.1016/j.cyto.2020.155252. Epub 2020 Aug 17. Cytokine. 2020. PMID: 32818703 Free PMC article. - β-Catenin and TCFs/LEF signaling discordantly regulate IL-6 expression in astrocytes.
Robinson KF, Narasipura SD, Wallace J, Ritz EM, Al-Harthi L. Robinson KF, et al. Cell Commun Signal. 2020 Jun 16;18(1):93. doi: 10.1186/s12964-020-00565-2. Cell Commun Signal. 2020. PMID: 32546183 Free PMC article.
References
- Atcha FA, Munguia JE, Li TW, Hovanes K, Waterman ML (2003) A new beta-catenin-dependent activation domain in T cell factor. J Biol Chem 278: 16169–16175 - PubMed
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 - PubMed
- Chapman DL, Papaioannou VE (1998) Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391: 695–697 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases