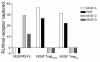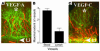VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment - PubMed (original) (raw)
VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment
Claus Cursiefen et al. J Clin Invest. 2004 Apr.
Abstract
Lymphangiogenesis, an important initial step in tumor metastasis and transplant sensitization, is mediated by the action of VEGF-C and -D on VEGFR3. In contrast, VEGF-A binds VEGFR1 and VEGFR2 and is an essential hemangiogenic factor. We re-evaluated the potential role of VEGF-A in lymphangiogenesis using a novel model in which both lymphangiogenesis and hemangiogenesis are induced in the normally avascular cornea. Administration of VEGF Trap, a receptor-based fusion protein that binds and neutralizes VEGF-A but not VEGF-C or -D, completely inhibited both hemangiogenesis and the outgrowth of LYVE-1(+) lymphatic vessels following injury. Furthermore, both lymphangiogenesis and hemangiogenesis were significantly reduced in mice transgenic for VEGF-A(164/164) or VEGF-A(188/188) (each of which expresses only one of the three principle VEGF-A isoforms). Because VEGF-A is chemotactic for macrophages and we demonstrate here that macrophages in inflamed corneas release lymphangiogenic VEGF-C/VEGF-D, we evaluated the possibility that macrophage recruitment plays a role in VEGF-A-mediated lymphangiogenesis. Either systemic depletion of all bone marrow-derived cells (by irradiation) or local depletion of macrophages in the cornea (using clodronate liposomes) prior to injury significantly inhibited both hemangiogenesis and lymphangiogenesis. We conclude that VEGF-A recruitment of monocytes/macrophages plays a crucial role in inducing inflammatory neovascularization by supplying/amplifying signals essential for pathological hemangiogenesis and lymphangiogenesis.
Figures
Figure 1
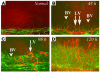
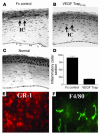
Concomitant induction of HA and lymphangiogenesis in inflammatory corneal neovascularization. (A–F) Seven days after central, intrastromal suture placement (A), a robust angiogenic response (A; blood vessel [BV]) in combination with an influx of inflammatory cells (B [H&E] and C) can be seen biomicroscopically (A) and by using CD31 (PECAM1) immunostaining (D) of corneal flat mounts (green). The CD45+ inflammatory cell infiltrate (C) consists mainly of GR-1+ neutrophils (red) and F4/80+ macrophages (green). In addition to the CD31+++LYVE-1– blood vessels (D and E; green), there is parallel outgrowth of CD31+LYVE-1+++ lymphatic vessels (LV; D–F; red). Blood vessels do not react with the lymphatic vascular–specific hyaluronic acid receptor LYVE-1 (F). Magnification, ×20 (A), ×200 (B and F), ×400 (C and E), and ×100 (D).
Figure 2
Time course of early inflammatory HA and lymphangiogenesis. (A–D) In inflammatory corneal neovascularization, there is very early and parallel outgrowth of both blood vessels (green) as well as lymphatic vessels (red) from the limbal vascular arcade (bottom of each picture) toward the suture into the normally avascular cornea (top of each picture). Both vessel types sprout as early as 24 hours after injury and progress over time, with lymphatic vessels (red staining) often preceding blood vessels (green staining). Magnification, ×100.
Figure 3
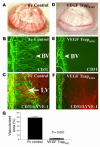
Neutralization of VEGF-A inhibits HA and lymphangiogenesis. (A–F) A molecular trap designed to bind VEGF-A (VEGF TrapR1R2) completely inhibits both HA and lymphangiogenesis within 1 week after injury. Whereas mice receiving an intraperitoneal injection of Fc protein at surgery (Fc control) display robust angiogenesis (A, slit-lamp picture; B, CD31 staining) and lymphangiogenesis (C, CD31 and LYVE-1 staining) 1 week later, mice treated with a single injection of VEGF TrapR1R2 do not show HA (D and E; blood vessels are green) or lymphangiogenesis (F; lymph vessels are red). Magnification, ×100 (C–F). (G) Morphometric analysis of the nearly complete inhibitory effect of VEGF Trap on both HA and lymphangiogenesis (P < 0.001). Magnification (A and B), ×20.
Figure 4
VEGF TrapR1R2 and VEGF TrapR1/A40 bind only VEGF-A/PlGF, not VEGF-C/VEGF-D. Biacore biochemical evaluation of binding of VEGF/PlGF growth factors to VEGF Traps and VEGF receptor chimeric proteins (VEGFR1-Fc, VEGFR2-Fc, and VEGFR3-Fc), demonstrates that VEGF TrapR1R2 and VEGF TrapR1/A40, used in this study, bind only VEGF-A/PlGF, not VEGF-C or -D. In contrast, VEGF-C and -D, but not VEGF-A/PlGF, bind to VEGFR3-Fc.
Figure 5
Importance of VEGF-A isoforms for lymphangiogenesis. (A–E) Double immunostaining CD31/LYVE-1 (blood vessels, green; lymphatic vessels, red) of corneal flat mounts of wild-type mice (A), VEGF-A164/164 transgenic mice (B), and VEGF-A188/188 transgenic mice (C) demonstrates significantly reduced HA (D; P < 0.05) and lymphangiogenesis (E; P < 0.05). Magnification, ×100 (A–C).
Figure 6
Effect of VEGF-A on lymphangiogenesis in corneal micropocket assay. (A–C) Pellets (*) containing 200 ng VEGF-A always induced a robust hemangiogenic response (A, green; Li, limbal vascular arcade [arrowhead]) and in 17 of 20 pellets in addition there was a mild to moderate lymphangiogenic response (red), which was significantly less compared with the hemangiogenic response (B). Panel C shows a representative and comparable effect by a VEGF-C pellet (200 ng). Magnification (A and C), ×100.
Figure 7
Anti-inflammatory effect of trapping VEGF-A. (A–C) Trapping of VEGF-A/PlGF using the molecular cytokine trap VEGF TrapR1R2 significantly reduces the recruitment of inflammatory cells into the cornea in the suture-induced neovascularization model. One week after surgery, control mice treated with Fc protein (Fc control) displayed a significant influx of inflammatory cells (IC and arrows) into the central corneal stroma (A). Trapping of VEGF-A significantly reduces this influx (B; and C, normal cornea). (D) Trapping of VEGF-A reduces stromal inflammatory cells by about 80% (P < 0.01). (E and F) The inflammatory cells found in the corneal stroma 7 days after suture placement and Fc treatment (controls) are overwhelmingly GR-1+ neutrophils (E, red) and less commonly, F4/80+CD11b+ macrophages (F, green). Magnification, ×100 (A–C) and ×400 (E and F).
Figure 8
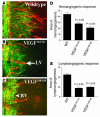
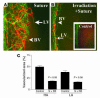
Bone marrow–derived cells mediate inflammation-associated lymphangiogenesis. (A–C) Depletion of bone marrow–derived cells induces a parallel inhibition of both HA and lymphangiogenesis in response to corneal inflammatory stimuli (blood vessels, green; lymphatic vessels, red). (A) Seven days after suture placement, control mice display parallel outgrowths of blood and lymphatic vessels from the limbal vascular arcade (left). (B and C) A single whole-body irradiation causes significant parallel inhibition of both HA and lymphangiogenesis. Inset in B shows a representative area of a normal limbal vascular arcade without vessel outgrowth. In C, controls are compared to irradiated mice (S + Rx); P < 0.05. Magnification (A and B), ×100.
Figure 9
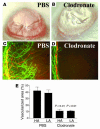
Macrophages are essential for pathological HA and lymphangiogenesis. (A and C) PBS-treated controls. (B and D) Mice that received subconjunctival clodronate liposomes. Magnification, ×100 (C and D). (E) Depletion of macrophages inhibits both HA and lymphangiogenesis (LA) in inflammatory neovascularization (P < 0.01). Magnification (A and B), ×20.
Figure 10
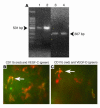
Macrophages in inflamed corneas express both VEGF-C and -D. (A) Cultivated, bone marrow–derived macrophages from BALB/c mice transcribe VEGF-C and -D mRNA 1 week after seeding. 1, VEGF-C positive control; 2, mouse bone marrow–derived macrophage VEGF-C; 3, VEGF-D positive control; 4: mouse bone marrow–derived macrophage VEGF-D. (B) Expression of VEGF-C (green) in red-stained CD11b+ macrophages in an inflamed cornea 48 hours after injury. (C) Expression of VEGF-D (green) in red-stained CD11b+ macrophages in an inflamed cornea 48 hours after injury. Arrows indicate a representative macrophage. Magnification (B and C), ×600.
Figure 11
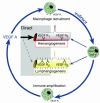
Proposed concept of an (indirect) lymphangiogenic role of VEGF-A via recruitment of bone marrow–derived macrophages, which in turn can release both hemangiogenic and lymphangiogenic growth factors. Macrophages seem to be important for immune amplification, leading to pathological HA and lymphangiogenesis.
Similar articles
- VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment.
Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. Murakami M, et al. Arterioscler Thromb Vasc Biol. 2008 Apr;28(4):658-64. doi: 10.1161/ATVBAHA.107.150433. Epub 2008 Jan 3. Arterioscler Thromb Vasc Biol. 2008. PMID: 18174461 - CXCL12/CXCR4 axis regulates neovascularization and lymphangiogenesis in sutured corneas in mice.
Du LL, Liu P. Du LL, et al. Mol Med Rep. 2016 Jun;13(6):4987-94. doi: 10.3892/mmr.2016.5179. Epub 2016 Apr 25. Mol Med Rep. 2016. PMID: 27121088 Free PMC article. - Blockade of the VEGF isoforms in inflammatory corneal hemangiogenesis and lymphangiogenesis.
Lipp M, Bucher F, Parthasarathy A, Hos D, Onderka J, Cursiefen C, Bock F. Lipp M, et al. Graefes Arch Clin Exp Ophthalmol. 2014 Jun;252(6):943-9. doi: 10.1007/s00417-014-2626-2. Epub 2014 Apr 15. Graefes Arch Clin Exp Ophthalmol. 2014. PMID: 24728466 - Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea.
Patnam M, Dommaraju SR, Masood F, Herbst P, Chang JH, Hu WY, Rosenblatt MI, Azar DT. Patnam M, et al. Cells. 2023 Jan 14;12(2):319. doi: 10.3390/cells12020319. Cells. 2023. PMID: 36672254 Free PMC article. Review. - Corneal lymphangiogenesis: implications in immunity.
Patel SP, Dana R. Patel SP, et al. Semin Ophthalmol. 2009 May-Jun;24(3):135-8. doi: 10.1080/08820530902801320. Semin Ophthalmol. 2009. PMID: 19437348 Review.
Cited by
- Inflammation in atherosclerosis: pathophysiology and mechanisms.
Ajoolabady A, Pratico D, Lin L, Mantzoros CS, Bahijri S, Tuomilehto J, Ren J. Ajoolabady A, et al. Cell Death Dis. 2024 Nov 11;15(11):817. doi: 10.1038/s41419-024-07166-8. Cell Death Dis. 2024. PMID: 39528464 Free PMC article. Review. - Inhibiting corneal transplantation rejection via lymphatic vessel ligation in a novel murine model.
Igarashi A, Hayashi T, Shimizu T, Yuda K, Yamagami S. Igarashi A, et al. Sci Rep. 2024 Oct 28;14(1):25692. doi: 10.1038/s41598-024-77160-9. Sci Rep. 2024. PMID: 39465339 Free PMC article. - Identifying methamphetamine use predictors in HIV infection: Immune-dopaminergic signatures in peripheral leukocytes and the role of COMT genotype.
Basova LV, Riley T, Franklin D, Delorme-Walker V, Lim WL, Grant I, Letendre SL, Iudicello JE, Cherner M, Ellis RJ, Marcondes MCG. Basova LV, et al. Brain Behav Immun Health. 2024 Oct 5;42:100873. doi: 10.1016/j.bbih.2024.100873. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39430881 Free PMC article. - Lymphangiogenesis: novel strategies to promote cutaneous wound healing.
Jian Y, Li Y, Zhang Y, Tang M, Deng M, Liu C, Cheng M, Xiao S, Deng C, Wei Z. Jian Y, et al. Burns Trauma. 2024 Sep 26;12:tkae040. doi: 10.1093/burnst/tkae040. eCollection 2024. Burns Trauma. 2024. PMID: 39328366 Free PMC article. Review. - Sustained and Efficient Delivery of Antivascular Endothelial Growth Factor by the Adeno-associated Virus for the Treatment of Corneal Neovascularization: An Outlook for Its Clinical Translation.
Xie M, Wang L, Deng Y, Ma K, Yin H, Zhang X, Xiang X, Tang J. Xie M, et al. J Ophthalmol. 2024 Sep 9;2024:5487973. doi: 10.1155/2024/5487973. eCollection 2024. J Ophthalmol. 2024. PMID: 39286553 Free PMC article. Review.
References
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. - PubMed
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. - PubMed
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer. 2002;2:727–739. - PubMed
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat. Rev. Cancer. 2002;2:573–583. - PubMed
- He Y, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer. Inst. 2002;94:819–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous