Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery - PubMed (original) (raw)
Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery
Ching-Pin Chang et al. J Clin Invest. 2004 Apr.
Abstract
Congenital obstructive nephropathy is the principal cause of renal failure in infants and children. The underlying molecular and cellular mechanisms of this disease, however, remain largely undetermined. We generated a mouse model of congenital obstructive nephropathy that resembles ureteropelvic junction obstruction in humans. In these mice, calcineurin function is removed by the selective deletion of Cnb1 in the mesenchyme of the developing urinary tract using the Cre/lox system. This deletion results in reduced proliferation in the smooth muscle cells and other mesenchymal cells in the developing urinary tract. Compromised cell proliferation causes abnormal development of the renal pelvis and ureter, leading to defective pyeloureteral peristalsis, progressive renal obstruction, and, eventually, fatal renal failure. Our study demonstrates that calcineurin is an essential signaling molecule in urinary tract development and is required for normal proliferation of the urinary tract mesenchymal cells in a cell-autonomous manner. These studies also emphasize the importance of functional obstruction, resulting from developmental abnormality, in causing congenital obstructive nephropathy.
Figures
Figure 1
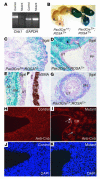
Deletion of calcineurin in cells expressing Pax3Cre. We generated mice carrying both the floxed-Cnb1 allele and the Pax3Cre transgene. In cells without Pax3Cre expression, the floxed-Cnb1 allele remained intact and functional. In cells with Pax3Cre expression, recombination occurred between the two loxP sites, leading to the deletion of exons 3–5 of Cnb1 and the inactivation of the gene.
Figure 2
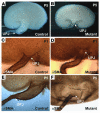
Conditional deletion of Cnb1 resulted in congenital obstructive nephropathy. (A–F) Urinary systems from controls (A, C, and E) and their littermate mutants (B, D, and F). Pictures were taken under different magnifications according to their size. The unit on the ruler is 1 mm. (G–L) H&E-stained paraffin sections from the controls (G, I, and K) and mutants (H, J, and L). Arrow in J points to a mildly dilated collecting tubule.
Figure 3
Pax3Cre directs _Cnb1_deletion in the metanephric and the ureteric mesenchyme. (A) RT-PCR shows the expression of _Cnb1_in the kidney and ureter of the mutant (_Pax3CreT/+;Cnb1F/Δ)_is reduced compared with that in the control (Pax3CreT/+;Cnb1F/+). GAPDH indicates equal loading. One hundred nanograms of total RNA was used in each lane. (B) Extensive Cre expression (revealed by lacZ expression; blue) was found in the kidney (K), ureter, and bladder (B) of the Pax3CreT/+;ROSAT/+ but not the Pax3Cre+/+;ROSAT/+ mice. (C–E and G) Samples from P1 Pax3CreT/+;ROSAT/+ mice. LacZ expression is evident in the glomeruli and tubules that are derived from the metanephric mesenchyme but not in the collecting duct system originated from the UB (C, ×20). The filled arrow in D points to the developing glomeruli. The open arrow points to one of the UB branches. In the developing renal pelvis, the SM layers (SM) and the adventitia (AD) express LacZ, while the UB-derived urothelium (UT) remains LacZ negative (D, ×40, and E, ×60). U, ureter; PP, papilla. The SM layers in the developing renal pelvic wall are illustrated by αSMA staining on a wild-type newborn sample (F, ×60). LacZ is also selectively expressed in the SM layers in the ureter but not in the urothelium (G, ×60). (H–K) Cnb1 protein can be detected by immunostaining in every cell in the control (H and J) but is absent in metanephric mesenchyme– and ureteric mesenchyme–derived structures in the mutant littermates (I and K). Cnb1 proteins remain in the UB-derived collecting duct (CD) system (open arrow) and the urothelium (filled arrow).
Figure 4
Defective pyeloureteral peristalsis in the mutant mice. These are a series of images taken at 2-second intervals during a peristaltic cycle in samples from a control (A–E) and a mutant littermate (F–J) at P5. The black bars indicate the change of length in the most proximal segment of the ureter.
Figure 5
Samples from controls (Pax3CreT/+;Cnb1F/+) (A, C, and E); and samples from their mutant littermates (Pax3CreT/+;Cnb1F/Δ) (
B
, D, and F). Arrows in each panel indicate the locations of the UPJs. (A and B) Hemisected kidneys. (C–F) UPJ areas immunostained with an αSMA antibody at P1 (C and D) and P5 (E and F) showing the developing renal pelvic extension in the controls (C and E) and the underdevelopment of such an extension in the same area in the mutants (D and F).
Figure 6
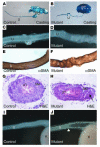
A defective ureteric wall may contribute to the defective peristalsis. (A and B) Replica moldings of the urinary tracts (from the pelvis to the distal end of the ureter) of the control (A) and the mutant (B) littermates at P5. (C and D) The ureteric wall is straight and smooth in the control (C) but irregular in the mutant (D). (E–H) The SM layers are disorganized in the mutants (F and H) but not the controls (E and G) at P5. Arrow in H points to the epithelial cells in the lumen of the cyst-like structure. (I and J) The mutant shown in J has hydroureter marked by the white triangle. Hydroureter has never been observed in the controls (I). PC, pelvicaliceal space.
Figure 7
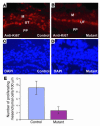
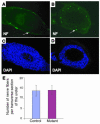
Calcineurin regulates cell proliferation in the developing renal pelvic wall. (A and B) Ki67 staining of the developing renal pelvic wall of control (A) and mutant (B) samples. (C and D) DAPI images of A and B, respectively. (E) The controls have significantly more proliferating mesenchymal cells along the developing renal pelvic wall. M, mesenchymal derivatives.
Figure 8
No developmental defects in the innervation of the urinary system in the mutants. (A and B) Immunostaining with an anti-neurofilament (NF) antibody revealed similar numbers of nerve fibers per transverse section between the control (A) and its littermate mutant at P1. (C and D) DAPI-stained sections from A and B, revealing the nuclei of the cells. (E) By counting only the nerve fibers occupying more than one-tenth the size of a nucleus of an average SMC, we found that the average numbers of nerve fibers are similar between the control samples and the mutant samples.
Figure 9
A model for the role of calcineurin (Cn) in the signaling events during urinary tract development. Deletion of Cnb1 in the mesenchyme along the urinary tract results in abnormal development of the renal pelvis and ureter by disrupting the proliferation of the mesenchymal cells and the development of the peristaltic machinery. Deletion of Shh in the urothelium (36) and deletion of Agtr1 and other genes in the renin-angiotensin axis (7, 37) also result in congenital obstructive phenotypes suggesting the possibility of interactions between these pathways. Ptch repression of Smo is relieved upon Shh binding, leading to the activation of Gli. p-NFAT, phosphorylated NFAT.
Comment in
- Functional obstruction: the renal pelvis rules.
Mendelsohn C. Mendelsohn C. J Clin Invest. 2004 Apr;113(7):957-9. doi: 10.1172/JCI21402. J Clin Invest. 2004. PMID: 15057300 Free PMC article.
Similar articles
- Tbx18 regulates the development of the ureteral mesenchyme.
Airik R, Bussen M, Singh MK, Petry M, Kispert A. Airik R, et al. J Clin Invest. 2006 Mar;116(3):663-74. doi: 10.1172/JCI26027. J Clin Invest. 2006. PMID: 16511601 Free PMC article. - Absence of canonical Smad signaling in ureteral and bladder mesenchyme causes ureteropelvic junction obstruction.
Tripathi P, Wang Y, Casey AM, Chen F. Tripathi P, et al. J Am Soc Nephrol. 2012 Apr;23(4):618-28. doi: 10.1681/ASN.2011060566. Epub 2012 Jan 26. J Am Soc Nephrol. 2012. PMID: 22282597 Free PMC article. - Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling.
Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Brenner-Anantharam A, et al. Development. 2007 May;134(10):1967-75. doi: 10.1242/dev.004234. Epub 2007 Apr 18. Development. 2007. PMID: 17442697 - Role of angiotensin in the development of the kidney and urinary tract.
Pope JC 4th, Nishimura H, Ichikawa I. Pope JC 4th, et al. Nephrologie. 1998;19(7):433-6. Nephrologie. 1998. PMID: 9857380 Review. - Genetic and developmental basis for urinary tract obstruction.
Chen F. Chen F. Pediatr Nephrol. 2009 Sep;24(9):1621-32. doi: 10.1007/s00467-008-1072-y. Epub 2008 Dec 16. Pediatr Nephrol. 2009. PMID: 19085015 Free PMC article. Review.
Cited by
- A new tool for conditional gene manipulation in a subset of keratin-expressing epithelia.
Wang Y, Guo Q, Casey A, Lin C, Chen F. Wang Y, et al. Genesis. 2012 Dec;50(12):899-907. doi: 10.1002/dvg.22046. Epub 2012 Aug 20. Genesis. 2012. PMID: 22764128 Free PMC article. - Tbx18 regulates the development of the ureteral mesenchyme.
Airik R, Bussen M, Singh MK, Petry M, Kispert A. Airik R, et al. J Clin Invest. 2006 Mar;116(3):663-74. doi: 10.1172/JCI26027. J Clin Invest. 2006. PMID: 16511601 Free PMC article. - Molecular anatomy of the kidney: what have we learned from gene expression and functional genomics?
Rumballe B, Georgas K, Wilkinson L, Little M. Rumballe B, et al. Pediatr Nephrol. 2010 Jun;25(6):1005-16. doi: 10.1007/s00467-009-1392-6. Epub 2010 Jan 5. Pediatr Nephrol. 2010. PMID: 20049614 Free PMC article. Review. - Non-invasive markers of ureteropelvic junction obstruction.
Decramer S, Bascands JL, Schanstra JP. Decramer S, et al. World J Urol. 2007 Oct;25(5):457-65. doi: 10.1007/s00345-007-0201-8. Epub 2007 Aug 14. World J Urol. 2007. PMID: 17701042 - Ablation of developing podocytes disrupts cellular interactions and nephrogenesis both inside and outside the glomerulus.
Jia Q, McDill BW, Sankarapandian B, Wu S, Liapis H, Holzman LB, Capecchi MR, Miner JH, Chen F. Jia Q, et al. Am J Physiol Renal Physiol. 2008 Dec;295(6):F1790-8. doi: 10.1152/ajprenal.90519.2008. Epub 2008 Oct 8. Am J Physiol Renal Physiol. 2008. PMID: 18842818 Free PMC article.
References
- Chevalier RL. Pathophysiology of obstructive nephropathy in the newborn. Semin. Nephrol. 1998;18:585–593. - PubMed
- Woolf, A.S., Winyard, P.J.D., Hermanns, M.M., and Welham, J.M. 2003. Maldevelopment of the human kidney and lower urinary tract: an overview. In The kidney: from normal development to congenital disease. P.D. Vize, A.S. Woolf, and J.B.L. Bard, editors. Academic Press Inc. San Diego, California, USA. 377–393.
- Grasso, M., and Gitlin, J. 2001. Ureteropelvic junction obstruction. eMedicine. [serial online]. http://www.emedicine.com/med/topic3074.htm.
- Zhang PL, Peters CA, Rosen S. Ureteropelvic junction obstruction: morphological and clinical studies. Pediatr. Nephrol. 2000;14:820–826. - PubMed
- Peters CA. Animal models of fetal renal disease. Prenat. Diagn. 2001;21:917–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DK064816/DK/NIDDK NIH HHS/United States
- R21 HL072092/HL/NHLBI NIH HHS/United States
- R21 DK64816/DK/NIDDK NIH HHS/United States
- R21 HL72092-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases