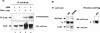Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction - PubMed (original) (raw)
Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction
Laurence Lévy et al. Mol Cell Biol. 2004 Apr.
Abstract
Lysine acetylation modulates the activities of nonhistone regulatory proteins and plays a critical role in the regulation of cellular gene transcription. In this study, we showed that the transcriptional coactivator p300 acetylated beta-catenin at lysine 345, located in arm repeat 6, in vitro and in vivo. Acetylation of this residue increased the affinity of beta-catenin for Tcf4, and the cellular Tcf4-bound pool of beta-catenin was significantly enriched in acetylated form. We demonstrated that the acetyltransferase activity of p300 was required for efficient activation of transcription mediated by beta-catenin/Tcf4 and that the cooperation between p300 and beta-catenin was severely reduced by the K345R mutation, implying that acetylation of beta-catenin plays a part in the coactivation of beta-catenin by p300. Interestingly, acetylation of beta-catenin had opposite, negative effects on the binding of beta-catenin to the androgen receptor. Our data suggest that acetylation of beta-catenin in the arm 6 domain regulates beta-catenin transcriptional activity by differentially modulating its affinity for Tcf4 and the androgen receptor. Thus, our results describe a new mechanism by which p300 might regulate beta-catenin transcriptional activity.
Figures
FIG. 1.
β-Catenin is acetylated in vivo. (A) 293 cells were transfected with T41A-β-catenin (5 μg) either alone or together with 5 μg of p300 or the empty vector. After metabolic labeling with 3H-labeled sodium acetate, proteins were immunoprecipitated (IP) with β-catenin antibodies and resolved on SDS-PAGE. The gel was then exposed to X-ray film (top panel). Immunoprecipitated proteins were analyzed by Western blotting (WB) with anti-β-catenin antibodies (bottom panel). (B) Endogenous β-catenin was immunoprecipitated from 293 or SW480 cells with anti-β-catenin antibodies. Control immunoprecipitation was performed with an anti-Flag antibody. The samples were resolved by SDS-PAGE, and acetylation was assessed with anti-acetyl-lysine antibodies (upper panel). The amount of β-catenin in the immunoprecipitates was determined with anti-β-catenin antibodies (bottom panel).
FIG. 2.
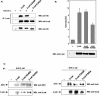
β-Catenin is acetylated at lysine residue K345 by p300. (A) Arm domain 6 of β-catenin is acetylated in vitro by p300. (Left panel) Schematic representation of GST-arm repeat constructs used in the in vitro acetylation assay. GST-full-length armadillo construct (GST-arm 1-12) or serial deletion constructs were incubated with GST-p300 recombinant protein and [14C]acetyl-coenzyme A. Reaction products were separated on SDS-10% PAGE. The gel was Coomassie blue-stained (bottom right panel) and then dried and exposed to X-ray film (upper right panel). (B) K345 is an acetyl acceptor in vitro. The amino acid sequence of the arm 6 domain contains two lysines (upper panel). GST-arm 1-12 (wt), GST-arm 1-12 K345R, or GST-arm 1-12 K354R was used for in vitro acetylation assays with GST-p300 recombinant protein. Reaction products were analyzed by SDS-PAGE and autoradiography. (C) In vitro acetylation assays with PCAF recombinant protein revealed acetylated PCAF and histone H3 but no acetylated form of β-catenin. (D) Lysine 345 is acetylated in vivo. HeLa cells were transfected with T41A-β-catenin or T41A-β-catenin K345R. Proteins were immunoprecipitated with β-catenin antibodies and resolved on SDS-PAGE. Blots were hybridized either with anti-acetyl-lysine antibodies or with anti-β-catenin antibodies. WB, Western blotting.
FIG. 3.
K345 of β-catenin is important for β-catenin/Tcf4 binding. (A) HeLa cells were cotransfected with either T41A-β-catenin, T41A-β-catenin K345R, or T41A-β-catenin K345A (5 μg) and with Tcf4-HA expression vector (2.5 μg). Proteins were extracted in lysis buffer A. Following coimmunoprecipitation (IP) with anti-β-catenin antibodies, proteins were resolved on SDS-PAGE, and Tcf4 was detected by Western blotting (WB) with anti-HA antibodies. The amounts of β-catenin in precipitates and Tcf4 in total cell lysates were shown by Western blotting with anti-β-catenin and anti-HA antibodies. (B) HeLa cells were cotransfected with the TOPFLASH reporter (0.1 μg) and T41A-β-catenin or the indicated K345 mutant (0.2 μg) or the empty vector. Luciferase activities were assayed 48 h after transfection. The basal activity of the TOPFLASH plasmid cotransfected with empty vector was arbitrarily set at 1, and other values were calculated accordingly. The data shown are the averages of three separate experiments done in duplicate. (Bottom) Expression levels of the different constructs determined by Western blotting with anti-β-catenin antibodies. (C) HeLa cells were transfected with T41A-β-catenin or the corresponding K345R or K345A mutant. Cellular extracts were prepared in buffer B, and coimmunoprecipitation assays were performed with anti-β-catenin monoclonal antibodies. Immunoprecipitates were resolved on SDS-PAGE, and blots were hybridized with either APC (left) or Axin (right panel) antibodies. Immunoprecipitated β-catenin levels were shown by Western blotting with anti-β-catenin antibodies.
FIG. 4.
Acetylation of K345 increases the affinity of β-catenin for Tcf4. (A) Recombinant GST-arm 1-12 or GST-arm 1-12 K345R or histone H3 was incubated with recombinant p300 protein in the presence or absence of [14C]acetyl-coenzyme A. Acetylated proteins were analyzed by SDS-PAGE and autoradiography. Coomassie blue staining confirmed that equivalent amounts of proteins were loaded. (B) For EMSA, recombinant proteins were eluted from the beads, and identical amounts of proteins were incubated with in vitro-translated Tcf4 and 32P-labeled Tcf probe. Reactions were resolved on 4% acrylamide gels (left panel). The relative intensities of β-catenin/Tcf4/DNA complexes were quantified with a PhosphorImager and are graphically depicted in the right-hand panel.
FIG. 5.
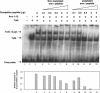
Acetylated arm 6 peptide competes for β-catenin/Tcf binding more efficiently than its nonacetylated counterpart. Acetylated or nonacetylated arm 6 peptide at increasing concentrations was incubated with in vitro-translated Tcf4. GST-arm 1-12 and 32P-labeled Tcf probe were added to the reaction for EMSA analysis, followed by autoradiography. The relative intensities of the β-catenin/Tcf4/DNA complex in the corresponding lanes were quantified by the Syngene Photo Image System (Syngene, Cambridge, United Kingdom) and are shown in bar graphs in the bottom panel.
FIG. 6.
Acetylated β-catenin associates preferentially with Tcf4. 293 cells were transfected with 5 μg of HA-Tcf4 or empty vector. Proteins were extracted in lysis buffer B, and in a first step, Tcf4 and bound proteins were coimmunoprecipitated with anti-HA antibodies. Both the immunoprecipitated (IP) fraction and the supernatant were recovered and used for a second round of immunoprecipitation with anti-β-catenin antibodies. Proteins were resolved on SDS-PAGE and analyzed by Western blotting (WB) with anti-acetyl-lysine antibodies (lanes 1 to 4) or anti-β-catenin antibodies (lanes 5 to 8). The ratios of acetylated β-catenin to total β-catenin were measured with Image Gauge software (Fuji) and are presented in graphic form. Tcf4 levels in the immunoprecipitates were determined by anti-HA Western blotting (lanes 9 and 10). Analysis of cell lysates by Western blotting with antiactin antibodies confirmed that equivalent amounts of proteins were used for immunoprecipitation assays (bottom).
FIG. 7.
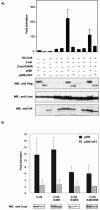
Cooperation between p300 and β-catenin is severely reduced by K345R mutation. (A) 293 cells were cotransfected with 0.1 μg of TOPFLASH reporter plasmid and either empty pCDNA3 vector or expression vectors for HA-Tcf4 (0.1 μg), T41A-β-catenin (0.25 μg), T41A-β-catenin K345R (0.25 mg), p300 (1.5 μg), and/or p300 ΔHAT (1.5 μg) as indicated. Luciferase activities were determined 48 h posttransfection. The basal TOPFLASH activity was set at 1, and data shown are the averages of three independent experiments performed in duplicate. WB, Western blotting. (Bottom panel) The expression levels of β-catenin, Tcf4, p300, and p300 ΔHAT were revealed by Western blotting with anti-β-catenin, anti-HA, and anti-Flag antibodies, respectively. (B) 293 cells were transfected with TOPFLASH and HA-Tcf4 (0.1 μg) and with either empty pCDNA3 vector or expression vectors for T41A-β-catenin or the indicated lysine mutants (0.25 μg), in combination or not with p300 or p300 ΔHAT (1.5 μg) as indicated. For each β-catenin mutant, TOPFLASH activity in cells cotransfectedwith Tcf4 and empty vector was arbitrarily set at 1. Data shown represent the activation by p300 or p300 ΔHAT for each β-catenin mutant. Values are the averages of three independent experiments performed in duplicate. The expression levels of the different β-catenin constructs in the presence of the coactivator are shown in the insets (bottom).
FIG. 8.
Acetylation of lysine 345 specifically increases the affinity of β-catenin for Tcf4. In vitro-translated Tcf4 (upper panels) and AR (lower panels) were incubated with immobilized GST-arm 1-12 in the presence of increasing amounts of acetylated or nonacetylated arm 6 peptide. Bound proteins were resolved on SDS-PAGE and analyzed by autoradiography.
Similar articles
- Acetylation-mediated transcriptional activation of the ETS protein ER81 by p300, P/CAF, and HER2/Neu.
Goel A, Janknecht R. Goel A, et al. Mol Cell Biol. 2003 Sep;23(17):6243-54. doi: 10.1128/MCB.23.17.6243-6254.2003. Mol Cell Biol. 2003. PMID: 12917345 Free PMC article. - Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin.
Labalette C, Renard CA, Neuveut C, Buendia MA, Wei Y. Labalette C, et al. Mol Cell Biol. 2004 Dec;24(24):10689-702. doi: 10.1128/MCB.24.24.10689-10702.2004. Mol Cell Biol. 2004. PMID: 15572674 Free PMC article. - Acetylation of the BETA2 transcription factor by p300-associated factor is important in insulin gene expression.
Qiu Y, Guo M, Huang S, Stein R. Qiu Y, et al. J Biol Chem. 2004 Mar 12;279(11):9796-802. doi: 10.1074/jbc.M307577200. Epub 2003 Dec 30. J Biol Chem. 2004. PMID: 14701848 - Wnt breakers in colon cancer.
Clevers H. Clevers H. Cancer Cell. 2004 Jan;5(1):5-6. doi: 10.1016/s1535-6108(03)00339-8. Cancer Cell. 2004. PMID: 14749120 Review. - Acetylation of general transcription factors by histone acetyltransferases.
Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H. Imhof A, et al. Curr Biol. 1997 Sep 1;7(9):689-92. doi: 10.1016/s0960-9822(06)00296-x. Curr Biol. 1997. PMID: 9285713 Review.
Cited by
- SIRT1 Mediates the Antagonism of Wnt/β-Catenin Pathway by Vitamin D in Colon Carcinoma Cells.
García-Martínez JM, Chocarro-Calvo A, Martínez-Useros J, Regueira-Acebedo N, Fernández-Aceñero MJ, Muñoz A, Larriba MJ, García-Jiménez C. García-Martínez JM, et al. Int J Biol Sci. 2024 Oct 7;20(14):5495-5509. doi: 10.7150/ijbs.95875. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494323 Free PMC article. - The role and mechanism of protein post‑translational modification in vascular calcification (Review).
Wang D, Li Q, Xie C. Wang D, et al. Exp Ther Med. 2024 Sep 6;28(5):419. doi: 10.3892/etm.2024.12708. eCollection 2024 Nov. Exp Ther Med. 2024. PMID: 39301258 Free PMC article. Review. - Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis.
Zhu S, Chen W, Masson A, Li YP. Zhu S, et al. Cell Discov. 2024 Jul 2;10(1):71. doi: 10.1038/s41421-024-00689-6. Cell Discov. 2024. PMID: 38956429 Free PMC article. Review. - Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy.
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu Y, Dong Q, Wei X. Song P, et al. J Hematol Oncol. 2024 Jun 18;17(1):46. doi: 10.1186/s13045-024-01563-4. J Hematol Oncol. 2024. PMID: 38886806 Free PMC article. Review. - Proximal tubular FHL2, a novel downstream target of hypoxia inducible factor 1, is a protector against ischemic acute kidney injury.
Wang Y, Kuang Z, Xing X, Qiu Y, Zhang J, Shao D, Huang J, Dai C, He W. Wang Y, et al. Cell Mol Life Sci. 2024 May 30;81(1):244. doi: 10.1007/s00018-024-05289-x. Cell Mol Life Sci. 2024. PMID: 38814462 Free PMC article.
References
- Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. - PubMed
- Bienz, M., and H. Clevers. 2003. Armadillo/beta-catenin signals in the nucleus-proof beyond a reasonable doubt? Nat. Cell Biol. 5:179-182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous