The presynaptic active zone protein RIM1alpha is critical for normal learning and memory - PubMed (original) (raw)
Comparative Study
The presynaptic active zone protein RIM1alpha is critical for normal learning and memory
Craig M Powell et al. Neuron. 2004.
Abstract
The active zone protein RIM1alpha is required both for maintaining normal probability of neurotransmitter release and for long-term presynaptic potentiation at brain synapses. We now demonstrate that RIM1alpha(-/-) mice exhibit normal coordination and anxiety-related behaviors but display severely impaired learning and memory. Mice with a synaptotagmin 1 mutation, which selectively lowers release probability, and mice with Rab3A deletion, which selectively abolishes presynaptic long-term potentiation, do not exhibit this abnormality. Our data suggest that a decrease in release probability or a loss of presynaptic LTP alone is not sufficient to cause major behavioral alterations, but the combination of presynaptic abnormalities in RIM1alpha(-/-) mice severely alters learning and memory.
Figures
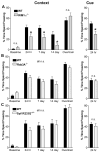
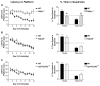
Figure 1. RIM1α−/− Mice Exhibit Impaired Fear Conditioning while Rab3A−/− and Syt1R233Q+/+ Do Not
(A) RIM1α−/− mice exhibit impaired fear conditioning. Context: Average percent time spent freezing in the context prior to training (baseline) and at various time points after training (24 hr, 7 day, and 14 day) in a one-trial fear conditioning paradigm (n = 23). “Overtrain” represents freezing 24 hr following 5 pairings of context/tone/shock. Results indicate a statistically significant decrease in contextual memory 24 hr after one-trial training that persists for at least 14 days. No difference was observed 24 hr after overtraining. Cue: Cue-dependent freezing tested 24 hr after one-trial training reveals a statistically significant decrease in fear memory (n = 23). (B) Rab3A−/− mice exhibit normal fear conditioning. Context and Cue: Context- and cue-dependent fear conditioning is not significantly different in Rab3A−/− mice versus wild-type under any condition (n = 23). (C) Syt1R233Q+/+ mice exhibit normal fear conditioning. Context and Cue: Context- and cue-dependent fear conditioning is not significantly different in Syt1R233Q+/+ mice versus wild-type under any condition (n = 21) (*p < 0.05; n.s., p > 0.05 in this and all subsequent figures).
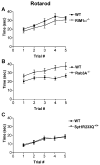
Figure 2. RIM1α−/− and Syt1R233Q+/+ Mice Have Normal Motor Coordination in the Rotarod Apparatus while Rab3A−/− Mice Display Slightly Better Ability
(A) RIM1α−/− mice exhibit normal motor coordination, staying on the accelerating rotarod as well as littermate controls (n = 20). (B) Rab3A−/− mice exhibit slightly better motor coordination compared to littermate controls, staying on the accelerating rotarod longer on average than littermate controls (n = 24). (C) Syt1R233Q+/+ mice exhibit normal motor coordination on the accelerating rotarod (n = 21).
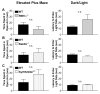
Figure 3. RIM1α−/−, Rab3A−/−, and Syt1R233Q+/+ Mice Display Normal Anxiety-like Behaviors in the Elevated Plus Maze and Dark/ Light Apparatus
(A) RIM1α−/− mice exhibit a nonsignificant trend toward increased anxiety in the elevated plus maze (n = 15) and dark/light tests (n = 24). (B and C) Rab3A−/− and Syt1R233Q+/+ mice displayed no difference in anxiety-like behavior on the elevated plus maze (Rab3A−/−, n = 12; Syt1R233Q+/+, n = 19) and dark/light tests (Rab3A−/−, n = 24; Syt1R233Q+/+, n = 20).
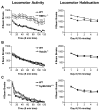
Figure 4. RIM1α−/− Mice Exhibit Increased Locomotor Response to Novelty while Rab3A−/− and Syt1R233Q+/+ Mice Exhibit Normal Locomotor Responses
(A) RIM1α−/− mice exhibit increased locomotor response to novelty. Locomotor Activity: RIM1α−/− mice displayed significantly increased locomotor activity over a 2 hr period in a fresh home cage. Both RIM1α−/− and wild-type mice displayed similar rates of habituation over the 2 hr period (n = 21). Locomotor Habituation: RIM1α−/− mice were more active than controls on the first day of a 10 min exposure to a novel chamber (n = 24). Over the next 4 days, RIM1α−/− mice habituated to activity levels equal to wild-type controls. Thus, the RIM1α−/− mice are not continually hyperactive, but exhibit abnormally increased locomotor activity in response to a novel environment. (B) Locomotor activity and habituation in Rab3A−/− mice are equivalent to wild-type controls. No significant difference was observed between Rab3A−/− mice and littermate controls on either locomotor activity (n = 24) or locomotor habituation (n = 24). (C) Locomtor activity and habituation in Syt1R233Q+/+ mice are equivalent to wild-type controls. No significant difference was observed in Syt1R233Q+/+ mice and littermate controls on locomotor activity (n = 19) or locomotor habituation (n = 20). The y axis on locomotor activity for the Syt1R233Q+/+ mice differs from that of Rab3A−/− and RIM1α−/− mice because Syt1R233Q+/+ mice were tested using more widely spaced photocells to measure activity. The apparatus, photocells, and absolute activity measurements were the same for locomotor habituation among the three lines of mice.
Figure 5. RIM1α−/− Mice Are Deficient in a Spatial Learning Task
(A) RIM1α−/− mice are impaired in spatial learning in the Morris water maze. Latency to Platform: RIM1α−/− mice do not decrease their latency to reach the submerged platform over 12 days of training (n = 12). Littermate wild-type controls do decrease their latency to reach the submerged platform to asymptotic levels by about day 7. % Time in Quadrants: A probe trial was performed on day 12 (n = 12). RIM1α−/− mice spent an equal percentage of time in the target quadrant (in which the submerged platform had been located) as in the quadrant opposite the target quadrant. Littermate controls showed a clear preference for the target quadrant during the probe test and spent significantly more time in the target quadrant than did the RIM1α−/− mice. (B) Rab3A−/− mice show normal spatial learning in the Morris water maze. Latency to Platform: Rab3A−/− mice displayed a learning curve equal to that of littermate controls (n = 12). % Time in Quadrants: Rab3A−/− mice and littermate controls showed equal preference for the target quadrant in the probe trial on day 12 (n = 12). (C) Syt1R233Q+/+ mice show normal spatial learning in the Morris water maze. Latency to Platform: The Syt1R233Q+/+ learning curve was not significantly different from that of littermate controls (n = 14). % Time in Quadrants: Equal preference for the target quadrant was observed for Syt1R233Q+/+ mice and littermate controls (n = 14).
Similar articles
- Deficits in memory and motor performance in synaptotagmin IV mutant mice.
Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR. Ferguson GD, et al. Proc Natl Acad Sci U S A. 2000 May 9;97(10):5598-603. doi: 10.1073/pnas.100104597. Proc Natl Acad Sci U S A. 2000. PMID: 10792055 Free PMC article. - Hippocalcin-deficient mice display a defect in cAMP response element-binding protein activation associated with impaired spatial and associative memory.
Kobayashi M, Masaki T, Hori K, Masuo Y, Miyamoto M, Tsubokawa H, Noguchi H, Nomura M, Takamatsu K. Kobayashi M, et al. Neuroscience. 2005;133(2):471-84. doi: 10.1016/j.neuroscience.2005.02.034. Neuroscience. 2005. PMID: 15878804 - [A presynaptic Ca(2+)-binding protein--synaptotagmin].
Qiang M, Qiao JT, Wu FM. Qiang M, et al. Sheng Li Ke Xue Jin Zhan. 1996 Jul;27(3):268-70. Sheng Li Ke Xue Jin Zhan. 1996. PMID: 9772372 Review. Chinese. No abstract available. - RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion.
Geppert M, Südhof TC. Geppert M, et al. Annu Rev Neurosci. 1998;21:75-95. doi: 10.1146/annurev.neuro.21.1.75. Annu Rev Neurosci. 1998. PMID: 9530492 Review.
Cited by
- Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch.
Lu J, Machius M, Dulubova I, Dai H, Südhof TC, Tomchick DR, Rizo J. Lu J, et al. PLoS Biol. 2006 Jul;4(7):e192. doi: 10.1371/journal.pbio.0040192. PLoS Biol. 2006. PMID: 16732694 Free PMC article. - Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons.
Wang J, Lv X, Wu Y, Xu T, Jiao M, Yang R, Li X, Chen M, Yan Y, Chen C, Dong W, Yang W, Zhuo M, Chen T, Luo J, Qiu S. Wang J, et al. Nat Commun. 2018 Jun 11;9(1):2267. doi: 10.1038/s41467-018-04672-0. Nat Commun. 2018. PMID: 29891949 Free PMC article. - Importance of glutamine in synaptic vesicles revealed by functional studies of SLC6A17 and its mutations pathogenic for intellectual disability.
Jia X, Zhu J, Bian X, Liu S, Yu S, Liang W, Jiang L, Mao R, Zhang W, Rao Y. Jia X, et al. Elife. 2023 Jul 13;12:RP86972. doi: 10.7554/eLife.86972. Elife. 2023. PMID: 37440432 Free PMC article. - Crystal structure of the RIM1alpha C2B domain at 1.7 A resolution.
Guan R, Dai H, Tomchick DR, Dulubova I, Machius M, Südhof TC, Rizo J. Guan R, et al. Biochemistry. 2007 Aug 7;46(31):8988-98. doi: 10.1021/bi700698a. Epub 2007 Jul 14. Biochemistry. 2007. PMID: 17630786 Free PMC article. - Kctd13 deletion reduces synaptic transmission via increased RhoA.
Escamilla CO, Filonova I, Walker AK, Xuan ZX, Holehonnur R, Espinosa F, Liu S, Thyme SB, López-García IA, Mendoza DB, Usui N, Ellegood J, Eisch AJ, Konopka G, Lerch JP, Schier AF, Speed HE, Powell CM. Escamilla CO, et al. Nature. 2017 Nov 9;551(7679):227-231. doi: 10.1038/nature24470. Epub 2017 Nov 1. Nature. 2017. PMID: 29088697 Free PMC article.
References
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. - PubMed
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. - PubMed
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. - PubMed
- Chapouthier G, Venault P. GABA-A receptor complex and memory processes. Curr Top Med Chem. 2002;2:841–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 MH065975-04/MH/NIMH NIH HHS/United States
- P50MH66172/MH/NIMH NIH HHS/United States
- K08 MH065975/MH/NIMH NIH HHS/United States
- K08MH65975/MH/NIMH NIH HHS/United States
- K08 MH065975-05/MH/NIMH NIH HHS/United States
- P50 MH066172/MH/NIMH NIH HHS/United States
- K08 MH065975-03/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases