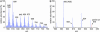Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration - PubMed (original) (raw)
Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration
Roxana A Radu et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Recessive Stargardt's macular degeneration is a blinding disease of children caused by mutations in the ABCA4 (ABCR) gene. Mice with a knockout mutation in abcr accumulate toxic lipofuscin pigments in ocular tissues, similar to affected humans. The major fluorophore of lipofuscin is the bis-retinoid, N-retinylidene-N-retinylethanolamine (A2E). In the current study, we sought to define the effect of increasing light on A2E accumulation. We crossed the abcr(-/-) mutation onto an albino background. The retinoid profiles in albino mice indicated higher retinal illuminance than in pigmented mice exposed to similar ambient light. Unexpectedly, A2E levels were not higher in the albino mice. Also, A2E levels in abcr(-/-) mice reared under cyclic light at 30, 120, or 1,700 lux were similar. Thus, increased retinal illuminance was not correlated with higher A2E. A2E has been shown to undergo light-dependent oxidation to yield a series of A2E epoxides or oxiranes. These oxiranes react with DNA in vitro, suggesting a potential mechanism for A2E cytotoxicity. We analyzed ocular tissues from abcr(-/-) mice for A2E oxiranes by mass spectrometry. Unlike A2E, the oxiranes were more abundant in albino vs. pigmented abcr(-/-) mice, and in abcr(-/-) mice exposed to increasing ambient light. These observations suggest that both the biosynthesis of A2E and its conversion to oxiranes are accelerated by light. Finally, we showed that the formation of A2E oxiranes is strongly suppressed by treating the abcr(-/-) mice with Accutane (isotretinoin), an inhibitor of rhodopsin regeneration.
Figures
Fig. 1.
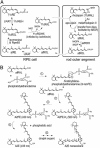
Retinoid pathways in the retina and RPE. (A) Visual cycle mediating rhodopsin regeneration. Absorption of a photon (hv) by a rhodopsin molecule in a rod outer segment disk induces photoisomerization of the 11_c_RAL chromophore, yielding activated metarhodopsin II. After several seconds, metarhodopsin II decays to yield apo-rhodopsin and free _at_RAL. ABCR functions to accelerate removal of _at_RAL from the interior of outer segment discs to the cytoplasmic space by flipping _N-_ret-PE (5). The _at_RAL is subsequently reduced to _at_ROL or vitamin A by _all_-_trans_-retinol dehydrogenase. The _at_ROL is released from the outer segment and taken up by an adjacent RPE cell where it is esterified by lecithin retinol acyl transferase (LRAT) to form an _at_RE. Chemical isomerization is effected by an isomerase that uses _at_REs as a substrate, in conjunction with Rpe65 (46). The resulting 11_c_ROL is oxidized by 11_c_RDH to form 11_c_RAL chromophore. 11_c_RDH is inhibited by isotretinoin with a _K_i of ≈0.1 μM (40, 47). 11_c_ROL may also serve as a substrate for LRAT to form 11-_cis_-retinyl esters. The final step is recombination of 11_c_RAL with aporhodopsin in the outer segment to form a new molecule of light-sensitive rhodopsin. (B) Synthesis of A2E. After light exposure, newly released _at_RAL condenses reversibly with phosphatidylethanolamine to _N_-ret-PE (step 1). Rarely, a second molecule of _at_RAL will condense with _N-_ret-PE to form A2PE-H2 (step 2). The wavelength of maximal absorption (λmax) for A2PE-H2 is 500 nm. Within the acidic and oxidizing environment of RPE phagolysosomes, A2PE-H2 is oxidized to A2PE (λmax = 430 nm) (step 3). Hydrolysis of the phosphate ester yields A2E (λmax = 435 nm) and phosphatidic acid (step 4) (10). Double bonds along the polyene chains of A2E may react with singlet oxygen to form a series of one to nine (shown) oxiranes (step 5).
Fig. 3.
A2E and A2PE-H2 in abcr_–/_– eyes. (A) Representative chromatogram of a phospholipid extract from a pigmented abcr_–/_– eyecup showing absorbance at 435 nm. (Inset) UV spectra acquired from the peaks labeled A2E and A2PE-H2. Note the λmax of A2E at 435 and A2PE-H2 at 500 nm. (B) Quantitation of A2E (pmol per eye) and A2PE-H2 (milli-absorbance unit per eye at 500 nm) in eyecups from 3-mo-old pigmented (dark blue bars) and albino (cyan bars) abcr_–/_– mice raised under cyclic light at 30 lux. Error bars show standard deviations (n = 4).
Fig. 2.
Visual retinoids in pigmented and albino mice. (A) Representative HPLC chromatogram of a retinoid extract from the eyecup of a 2-mo-old pigmented wild-type mouse showing absorbance at 340 nm. Labeled peaks corresponding to _at_REs, _at_ROL, and the _syn_- and _anti-_oximes of 11_c_RAL (11_c_Rox) and _at_RAL (_at_Rox) were confirmed by spectral analysis. (B) Chromatogram of authentic retinoid standards at 340-nm absorbance. (C) Quantitation of retinoids from eyecups of 2- to 3-mo-old wild-type pigmented (dark blue bars) and albino (cyan bars) mice, light adapted at 30 lux. Values for the indicated retinoids are shown in pmol per eye. (D) Quantitation of retinoids from eyecups of 2- to 3-mo-old pigmented and albino abcr_–/_– mice light adapted at 30 lux. Error bars show standard deviations (n = 4).
Fig. 4.
Effects of light on A2E and A2PE-H2. Quantitation of A2E (magenta bars) in pmol per eye and A2PE-H2 (blue bars) in milli-absorbance unit per eye from 5-mo-old pigmented abcr_–/_– mice raised under cyclic light at 30, 120, or 1,700 lux. Error bars show standard deviations (n = 3).
Fig. 5.
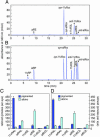
Identification of A2E oxiranes generated in vitro and in vivo by MS. (A) Full-scan MS in the range 580–750 m/z of oxiranes after chemical oxidation of in vitro synthesized A2E. Note A2E at 592 and the family of ions that increase by 16 mass units to nonaoxirane at 736. (B) Product-ion spectrum showing fragmentation of a 640-m/z parent ion in a phospholipid extract of 7-mo-old abcr_–/_– eyecups. Note the formation of bisoxirane (624), monooxirane (608), and A2E (592).
Fig. 6.
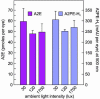
A2E and oxiranes in abcr_–/_– mice at different retinal illuminance. (A) Quantitation of A2E and total oxiranes in eyecups from 7-mo-old abcr_–/_– pigmented (dark blue bars) and albino (cyan bars) mice raised under cyclic light at 30 lux determined by LC-MS (n = 4). (B) Quantitation of A2E and total oxiranes in 7-mo-old abcr_–/_– pigmented mice raised under cyclic light at 30 (dark green bars) or 120 lux (light green bars). Values are expressed as ion intensities relative to a lysophosphatidylcholine internal standard. Error bars show standard deviations (n = 3). Note the similar levels of A2E and elevated oxiranes with increased illuminance in both experiments.
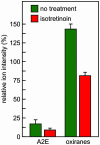
Fig. 7.
Effect of treatment with isotretinoin on A2E and oxiranes in abcr_–/_– mice. Quantitation of A2E and total oxiranes in eyecups from pigmented 4-mo-old abcr_–/_– mice raised under cyclic light at 30 lux and treated (red bars) or not treated (green bars) with isotretinoin for 2 mo. Error bars show standard deviations (n = 4). Note the reduction of both A2E and oxiranes in the treated mice.
Similar articles
- Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration.
Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Radu RA, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4742-7. doi: 10.1073/pnas.0737855100. Epub 2003 Apr 1. Proc Natl Acad Sci U S A. 2003. PMID: 12671074 Free PMC article. - Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration.
Radu RA, Mata NL, Nusinowitz S, Liu X, Travis GH. Radu RA, et al. Novartis Found Symp. 2004;255:51-63; discussion 63-7, 177-8. Novartis Found Symp. 2004. PMID: 14750596 - Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration.
Mata NL, Weng J, Travis GH. Mata NL, et al. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7154-9. doi: 10.1073/pnas.130110497. Proc Natl Acad Sci U S A. 2000. PMID: 10852960 Free PMC article. - Stargardt's disease and the ABCR gene.
Westerfeld C, Mukai S. Westerfeld C, et al. Semin Ophthalmol. 2008 Jan-Feb;23(1):59-65. doi: 10.1080/08820530701745249. Semin Ophthalmol. 2008. PMID: 18214793 Review. - Macular Pigment Carotenoids and Bisretinoid A2E.
Arunkumar R, Bernstein PS. Arunkumar R, et al. Adv Exp Med Biol. 2023;1415:15-20. doi: 10.1007/978-3-031-27681-1_3. Adv Exp Med Biol. 2023. PMID: 37440008 Review.
Cited by
- Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport.
Coleman JA, Quazi F, Molday RS. Coleman JA, et al. Biochim Biophys Acta. 2013 Mar;1831(3):555-74. doi: 10.1016/j.bbalip.2012.10.006. Epub 2012 Oct 26. Biochim Biophys Acta. 2013. PMID: 23103747 Free PMC article. Review. - Membrane Attack Complex Mediates Retinal Pigment Epithelium Cell Death in Stargardt Macular Degeneration.
Ng ESY, Kady N, Hu J, Dave A, Jiang Z, Pei J, Gorin MB, Matynia A, Radu RA. Ng ESY, et al. Cells. 2022 Nov 2;11(21):3462. doi: 10.3390/cells11213462. Cells. 2022. PMID: 36359858 Free PMC article. - Functional roles of bestrophins in ocular epithelia.
Marmorstein AD, Cross HE, Peachey NS. Marmorstein AD, et al. Prog Retin Eye Res. 2009 May;28(3):206-26. doi: 10.1016/j.preteyeres.2009.04.004. Epub 2009 May 4. Prog Retin Eye Res. 2009. PMID: 19398034 Free PMC article. Review. - Intrinsic tissue fluorescence in an organotypic perfusion culture of the porcine ocular fundus exposed to blue light and free radicals.
Hammer M, Richter S, Kobuch K, Mata N, Schweitzer D. Hammer M, et al. Graefes Arch Clin Exp Ophthalmol. 2008 Jul;246(7):979-88. doi: 10.1007/s00417-008-0789-4. Epub 2008 Mar 20. Graefes Arch Clin Exp Ophthalmol. 2008. PMID: 18351374 - Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration.
Rezaie T, McKercher SR, Kosaka K, Seki M, Wheeler L, Viswanath V, Chun T, Joshi R, Valencia M, Sasaki S, Tozawa T, Satoh T, Lipton SA. Rezaie T, et al. Invest Ophthalmol Vis Sci. 2012 Nov 27;53(12):7847-54. doi: 10.1167/iovs.12-10793. Invest Ophthalmol Vis Sci. 2012. PMID: 23081978 Free PMC article.
References
- Lee, B. L. & Heckenlively, J. R. (1999) in Retina-Vitreous-Macula, eds. Guyer, D. R., Yannuzzi, L. A., Chang, S., Shields, J. A. & Green, W. R. (Saunders, Darien, IL), pp. 978–988.
- Eagle, R. C., Jr., Lucier, A. C., Bernardino, V. B., Jr., & Yanoff, M. (1980) Ophthalmology 87, 1189–1200. - PubMed
- Birnbach, C. D., Jarvelainen, M., Possin, D. E. & Milam, A. H. (1994) Ophthalmology 101, 1211–1219. - PubMed
- Steinberg, R. H. (1985) Doc. Ophthalmol. 60, 327–346. - PubMed
- Weng, J., Mata, N. L., Azarian, S. M., Tzekov, R. T., Birch, D. G. & Travis, G. H. (1999) Cell 98, 13–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous