Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice - PubMed (original) (raw)
Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice
Sandra Luikenhuis et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Mutations in MECP2 are the cause of Rett syndrome (RTT) in humans, a neurodevelopmental disorder that affects mainly girls. MeCP2 is a protein that binds CpG dinucleotides and is thought to act as a global transcriptional repressor. It is highly expressed in neurons, but not in glia, of the postnatal brain. The timing of MeCP2 activation correlates with the maturation of the central nervous system, and recent reports suggest that MeCP2 may be involved in the formation of synaptic contacts and may function in activity-dependent neuronal gene expression. Deletion or targeted mutation of Mecp2 in mice leads to a Rett-like phenotype. Selective mutation of Mecp2 in postnatal neurons leads to a similar, although delayed, phenotype, suggesting that MeCP2 plays a role in postmitotic neurons. Here we test the hypothesis that the symptoms of RTT are exclusively caused by a neuronal MeCP2 deficiency by placing Mecp2 expression under the control of a neuron-specific promoter. Expression of the Mecp2 transgene in postmitotic neurons resulted in symptoms of severe motor dysfunction. Transgene expression in Mecp2 mutant mice, however, rescued the RTT phenotype.
Figures
Fig. 1.
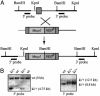
Targeting the MeCP2 cDNA to the tau locus. (A) Targeting strategy to insert the Mecp2 cDNA and the neomycin resistance marker (NEOR) into exon 1 of the tau locus. The upstream and downstream targeting arms are shown in light and dark gray, respectively. The locations of 5′ and 3′ external probes used for Southern blot analysis are indicated. (B) Southern blot analysis of targeted embryonic stem cell clones (ki/+) after digestion with _Bam_HI (Left) and _Kpn_I (Right). When hybridized with the 5′ external probe, wild-type clones display a 9-kb band. The correct targeting event results in a band-shift to 4.75 kb for the targeted allele. Hybridization with the 3′ external probe results in a 8.8-kb wild-type band and a 12.5-kb band for the targeted allele.
Fig. 2.
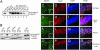
Expression of MeCP2 from the tau locus. (A) Immunoblot analysis of protein prepared from whole brain samples. As controls, 40 μg of protein were loaded from wild-type and Mecp2 mutant (Mecp2 KO) animals, and Mecp2 mutant animals heterozygous for the Tau-Mecp2 transgene (Rescue). The protein extract from a Mecp2 wild-type animal heterozygous for the transgene (Tau-Mecp2 ki/+) was loaded as serial dilutions as indicated. The Tau-MeCP2 fusion protein contains 31 aa of the Tau protein, which results in a band shift. (B) Endogenous MeCP2 (wild-type animal, wt) is highly expressed in the lung and spleen, and less in the liver, kidney, and heart. Tau-MeCP2 expression (rescued animal, R) is high in lung and kidney. Low-level expression is also detectable in the heart. In the liver and spleen Tau-MeCP2 is detectable only after long exposure times. (C_–_R) Tau-MeCP2 expression is neuron-specific in the brain and localizes to heterochromatic foci. Double labeled immuno-fluorescence of MeCP2 (green) and neuron-specific nuclear protein (NeuN, red) from wild-type (C_–_F), Mecp2 KO (G_–_J), rescued (K_–_N) and Tau-Mecp2 homozygous (Tau-Mecp2 ki/ki, O_–_R) hippocampi of adult animals are shown. Punctate MeCP2 staining is detectable in wild-type animals (C) as well as animals carrying the Tau-Mecp2 transgene (K and O). Insets in C, G, K, and O show enlargements of a small number of cells to illustrate MeCP2 staining. Endogenous MeCP2 as well as Tau-MeCP2 expression overlaps with NeuN immunoreactivity (F, N, and R). Only weak diffuse MeCP2 staining is present in Mecp2 null cells (G). Nuclear 4′,6-diamidino-2-phenylindole (DAPI) stain is shown in blue (E, I, M, and Q).
Fig. 3.
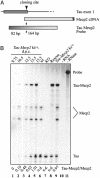
Timing of Tau-Mecp2 expression. (A) Probe design for the RNase protection assay. The probe overlaps the Tau-Mecp2 junction of the transgene and consists of 82 bp of Tau sequence and 164 bp of Mecp2 cDNA sequence (26 bp of exon 2 and 138 bp of exon 3). (B) RNase protection assay. RNA was extracted from embryos (9.75 dpc) and embryonic heads (10.5–15.5 dpc) of Mecp2 wild-type animals heterozygous for the Tau-Mecp2 transgene (lanes 1–5), or from brains of adult control animals (lanes 6–9). Lane 10 shows the no sample control, and lane 11 is 15% loading of the no RNase control. Wild-type Mecp2 RNA is detectable as two bands that probably represent different splice products (lanes 1–6 and 9). No Mecp2 signal is detectable in animals carrying the mutant Mecp2 allele that lacks exon 3 (lanes 7 and 8). The ratio of Tau-Mecp2 RNA expression to endogenous Mecp2 RNA as quantitated by phosphorimaging is given below the gel.
Fig. 4.
Expression of MeCP2 in postmitotic neurons rescues the RTT phenotype, but overexpression of MeCP2 is detrimental. (A) Rescued animals are indistinguishable from their wild-type littermates. Animals are shown at 8 weeks of age. (B) Mecp2 wild-type animals heterozygous for Tau-Mecp2 (Tau-Mecp2 ki/+) show normal physical development. Mecp2 overexpressing animals (Tau-Mecp2 ki/ki) have a normal birth weight, but progressively lose weight so that animals are up to 60% smaller than wild-type littermates at weaning age. (C) Neuronal expression of MeCP2 rescues the weight-loss phenotype. Mecp2 null animals start to lose weight at ≈5 weeks of age. The weight of rescued as well as Tau-Mecp2 ki/+ animals is comparable to that of wild-type littermates. (D) Rescued animals have a brain weight comparable to wild-type littermates. In contrast, Mecp2 mutant animals show a 15–18% reduction in brain weight. (E) Spontaneous activity of rescued animals is comparable with the activity of wild-type littermates as measured by an infrared beam activated movement detector.
Similar articles
- Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice.
Chen RZ, Akbarian S, Tudor M, Jaenisch R. Chen RZ, et al. Nat Genet. 2001 Mar;27(3):327-31. doi: 10.1038/85906. Nat Genet. 2001. PMID: 11242118 - Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2.
Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Giacometti E, et al. Proc Natl Acad Sci U S A. 2007 Feb 6;104(6):1931-6. doi: 10.1073/pnas.0610593104. Epub 2007 Jan 31. Proc Natl Acad Sci U S A. 2007. PMID: 17267601 Free PMC article. - Rett syndrome: the complex nature of a monogenic disease.
Renieri A, Meloni I, Longo I, Ariani F, Mari F, Pescucci C, Cambi F. Renieri A, et al. J Mol Med (Berl). 2003 Jun;81(6):346-54. doi: 10.1007/s00109-003-0444-9. Epub 2003 May 16. J Mol Med (Berl). 2003. PMID: 12750821 Review. - Quantitative localization of heterogeneous methyl-CpG-binding protein 2 (MeCP2) expression phenotypes in normal and Rett syndrome brain by laser scanning cytometry.
LaSalle JM, Goldstine J, Balmer D, Greco CM. LaSalle JM, et al. Hum Mol Genet. 2001 Aug 15;10(17):1729-40. doi: 10.1093/hmg/10.17.1729. Hum Mol Genet. 2001. PMID: 11532982 - The neurobiology of Rett syndrome.
Akbarian S. Akbarian S. Neuroscientist. 2003 Feb;9(1):57-63. doi: 10.1177/1073858402239591. Neuroscientist. 2003. PMID: 12580340 Review.
Cited by
- Rett syndrome: insights into genetic, molecular and circuit mechanisms.
Ip JPK, Mellios N, Sur M. Ip JPK, et al. Nat Rev Neurosci. 2018 Jun;19(6):368-382. doi: 10.1038/s41583-018-0006-3. Nat Rev Neurosci. 2018. PMID: 29740174 Free PMC article. Review. - Rett syndrome and MeCP2.
Liyanage VR, Rastegar M. Liyanage VR, et al. Neuromolecular Med. 2014 Jun;16(2):231-64. doi: 10.1007/s12017-014-8295-9. Epub 2014 Mar 11. Neuromolecular Med. 2014. PMID: 24615633 Free PMC article. Review. - Cell-type-specific repression by methyl-CpG-binding protein 2 is biased toward long genes.
Sugino K, Hempel CM, Okaty BW, Arnson HA, Kato S, Dani VS, Nelson SB. Sugino K, et al. J Neurosci. 2014 Sep 17;34(38):12877-83. doi: 10.1523/JNEUROSCI.2674-14.2014. J Neurosci. 2014. PMID: 25232122 Free PMC article. - Inhibitory Synaptic Influences on Developmental Motor Disorders.
Fogarty MJ. Fogarty MJ. Int J Mol Sci. 2023 Apr 9;24(8):6962. doi: 10.3390/ijms24086962. Int J Mol Sci. 2023. PMID: 37108127 Free PMC article. Review. - Total RNA Sequencing of Rett Syndrome Autopsy Samples Identifies the M4 Muscarinic Receptor as a Novel Therapeutic Target.
Gogliotti RG, Fisher NM, Stansley BJ, Jones CK, Lindsley CW, Conn PJ, Niswender CM. Gogliotti RG, et al. J Pharmacol Exp Ther. 2018 May;365(2):291-300. doi: 10.1124/jpet.117.246991. Epub 2018 Mar 9. J Pharmacol Exp Ther. 2018. PMID: 29523700 Free PMC article.
References
- Zoghbi, H. Y. (2003) Science 302, 826-830. - PubMed
- Kriaucionis, S. & Bird, A. (2003) Hum. Mol. Genet. 12, Spec. No. 2, R221-R227. - PubMed
- Amir, R. E., Van den Veyver, I. B., Wan, M., Tran, C. Q., Francke, U. & Zoghbi, H. Y. (1999) Nat. Genet. 23, 185-188. - PubMed
- Nan, X., Ng, H. H., Johnson, C. A., Laherty, C. D., Turner, B. M., Eisenman, R. N. & Bird, A. (1998) Nature 393, 386-389. - PubMed
- Shahbazian, M. D., Antalffy, B., Armstrong, D. L. & Zoghbi, H. Y. (2002) Hum. Mol. Genet. 11, 115-124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases