The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape - PubMed (original) (raw)
Review
The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape
Ignacio Algarra et al. Cancer Immunol Immunother. 2004 Oct.
Abstract
Tumor immune escape variants can be identified in human and experimental tumors. A variety of different strategies are used by tumor cells to avoid recognition by different immune effector mechanisms. Among these escape routes, alteration of MHC class I cell surface expression is one of the mechanisms most widely used by tumor cells. In this review we focus our attention on the T-cell immune selection of MHC class I-deficient tumor variants. Different altered MHC class I phenotypes that originate from multiple molecular mechanisms can be identified in human tumors. MHC-deficient tumor clones can escape T-cell immune responses, but are in theory more susceptible to NK-cell-mediated lysis. In this context, we also review the controversial issue of the aberrant expression of nonclassical HLA class I molecules, particularly HLA-G, in tumors. This expression may be relevant in tumor cells that have lost the capacity to interact with NK inhibitory receptors-namely, those tumor cells with no HLA-B or HLA-C expression. Most published studies have not analyzed these possibilities and do not provide information about the complete HLA-A, HLA-B, or HLA-C molecule profiles of the tumors studied. In contrast, HLA-E has been reported to be expressed in some tumor cell lines with very low HLA-A, HLA-B, and HLA-C expression, suggesting that HLA-E may indeed, in some cases, play a role by inhibiting NK lysis of cells that otherwise would be destroyed by NK cells. Finally, we provide evidence that the status of the immune system in the tumor-bearing animal is capable of defining the MHC profile of the tumor cells. In other words, MHC class I-negative metastatic colonies are produced in immunocompetent animals, and MHC class I-positive colonies in T-cell immunodeficient individuals.
Figures
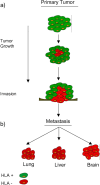
Fig. 1a
The primary tumor is composed originally of HLA-positive cells. During tumor development HLA-negative cells appear and are immunoselected by T-lymphocyte antitumor immune responses. b The metastatic nodes are composed of highly selected tumor clones with identical or sometimes different HLA class I deficiencies
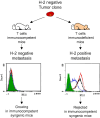
Fig. 2
The MHC class I phenotype of a metastatic tumor clone is dependent on the immune status of the host: metastatic nodes are MHC class I–negative in immunocompetent mice, and MHC class I–positive in T-cell–immunodeficient animals
Similar articles
- MHC class I antigens, immune surveillance, and tumor immune escape.
Garcia-Lora A, Algarra I, Garrido F. Garcia-Lora A, et al. J Cell Physiol. 2003 Jun;195(3):346-55. doi: 10.1002/jcp.10290. J Cell Physiol. 2003. PMID: 12704644 Review. - Involvement of HLA class I molecules in the immune escape of urologic tumors.
Carretero R, Gil-Julio H, Vázquez-Alonso F, Garrido F, Castiñeiras J, Cózar JM. Carretero R, et al. Actas Urol Esp. 2014 Apr;38(3):192-9. doi: 10.1016/j.acuro.2013.06.006. Epub 2013 Dec 7. Actas Urol Esp. 2014. PMID: 24315763 Review. English, Spanish. - Analysis of HLA-E expression in human tumors.
Marín R, Ruiz-Cabello F, Pedrinaci S, Méndez R, Jiménez P, Geraghty DE, Garrido F. Marín R, et al. Immunogenetics. 2003 Feb;54(11):767-75. doi: 10.1007/s00251-002-0526-9. Epub 2003 Jan 30. Immunogenetics. 2003. PMID: 12618909 - MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components.
Garcia-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. Garcia-Lora A, et al. Int J Cancer. 2003 Sep 10;106(4):521-527. doi: 10.1002/ijc.11241. Int J Cancer. 2003. PMID: 12845647 - MHC/HLA Class I Loss in Cancer Cells.
Garrido F. Garrido F. Adv Exp Med Biol. 2019;1151:15-78. doi: 10.1007/978-3-030-17864-2_2. Adv Exp Med Biol. 2019. PMID: 31140106 Review.
Cited by
- A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines.
Boegel S, Löwer M, Bukur T, Sahin U, Castle JC. Boegel S, et al. Oncoimmunology. 2014 Aug 3;3(8):e954893. doi: 10.4161/21624011.2014.954893. eCollection 2014. Oncoimmunology. 2014. PMID: 25960936 Free PMC article. - Sensitivity of Dendritic Cells to Microenvironment Signals.
Motta JM, Rumjanek VM. Motta JM, et al. J Immunol Res. 2016;2016:4753607. doi: 10.1155/2016/4753607. Epub 2016 Mar 21. J Immunol Res. 2016. PMID: 27088097 Free PMC article. Review. - Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma.
Kleinberg L, Flørenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, Nesland JM, Shih IeM, Davidson B. Kleinberg L, et al. Virchows Arch. 2006 Jul;449(1):31-9. doi: 10.1007/s00428-005-0144-7. Epub 2006 Mar 16. Virchows Arch. 2006. PMID: 16541284 - Natural Killer Cell Education Is Associated With a Distinct Glycolytic Profile.
Pfeifer C, Highton AJ, Peine S, Sauter J, Schmidt AH, Bunders MJ, Altfeld M, Körner C. Pfeifer C, et al. Front Immunol. 2018 Dec 19;9:3020. doi: 10.3389/fimmu.2018.03020. eCollection 2018. Front Immunol. 2018. PMID: 30619362 Free PMC article. - Surgery as a double-edged sword: a clinically feasible approach to overcome the metastasis-promoting effects of surgery by blunting stress and prostaglandin responses.
Benish M, Ben-Eliyahu S. Benish M, et al. Cancers (Basel). 2010 Nov 24;2(4):1929-51. doi: 10.3390/cancers2041929. Cancers (Basel). 2010. PMID: 24281210 Free PMC article.
References
- Algarra Int J Cancer. 1991;6:73.
- Boon Annu Rev Immunol. 1994;12:337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials