Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis - PubMed (original) (raw)
Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis
Sebastian Treusch et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Mucolipidosis type IV (MLIV) is an autosomal recessive lysosomal storage disease characterized by severe psychomotor retardation, achlorhydria, and ophthalmological abnormalities. Cells from several tissues in MLIV patients accumulate large vacuoles that are presumed to be lysosomes, but whose exact nature remains to be determined. Other defects include the deterioration of neuronal integrity in the retina and the cerebellum. MCOLN1, the gene mutated in MLIV patients, encodes a protein called h-mucolipin-1 that has six predicted transmembrane domains and functions as a Ca(2+)-permeable channel that is modulated by changes in Ca2+ concentration. CUP-5 is the Caenorhabditis elegans functional orthologue of h-mucolipin-1. Mutations in cup-5 result in the accumulation of large vacuoles in several cells, in increased cell death, and in embryonic lethality. We demonstrate here that CUP-5 functions in the biogenesis of lysosomes originating from hybrid organelles. We also show that at least two h-mucolipin family members rescue cup-5 mutant endocytic defects, indicating that there may be functional redundancy among the human proteins. Finally, we propose a model that relates the lysosome biogenesis defect in the absence of CUP-5/h-mucolipin-1 to cellular phenotypes in worms and in humans.
Figures
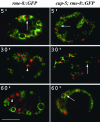
Fig. 1.
Time course of uptake of BSA-Rhod by WT or by cup-5(zu223) coelomocytes expressing RME-8::GFP. Confocal images of unc-36(e251); rme-8::GFP (Left) or cup-5(zu223) unc-36(e251); rme-8::GFP (Right) coelomocytes at the indicated times after BSA-Rhod injection into the body cavity of the respective worms. RME-8::GFP is green, and the BSA-Rhod is red. Large arrows indicate concentrations of the BSA-Rhod in compartments labeled with RME-8::GFP. Arrowheads indicate lysosomes that are not labeled with RME-8::GFP. (Bar, 5 μm in all images.)
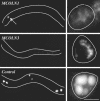
Fig. 3.
Morphological characterization of large vacuoles in coelomocytes of cup-5(ar465) worms. Shown are confocal images of WT or of cup-5(ar465) coelomocytes expressing the indicated GFP fusions (green) 24 h after BSA-Rhod (red) injection into the body cavity. Large arrows indicate large vacuoles, small arrows indicate lysosomes, arrowheads indicate late endosomes, the asterisk indicates heterogeneity in BSA-Rhod staining within the large vacuoles, and the small compartments stained by mannosidase II::GFP (MAN::GFP) are indicated with a G. Coelomocytes are outlined. (Bar, 5 μm in all images.)
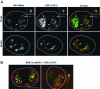
Fig. 2.
CUP-5 localization to nascent and to mature lysosomes. (A) Confocal images of individual coelomocytes expressing GFP::CUP-5 (green) after injection of BSA-Rhod (red) into the body cavity. Images were collected at the 15- or 60-min time points. Large arrows indicate late endosomes, small arrows indicate lysosomes, and arrowheads indicate overlap in staining on nascent lysosomes. (Inset) A higher-magnification view of an en face view of nascent lysosomes budding from a late endosome in another coelomocyte. (B) Confocal (Left) and deconvolution (Right) images of coelomocytes coexpressing RME-8::mRFP1 (red) and GFP::CUP-5 (green). Arrowheads indicate localization of CUP-5 to a microdomain on RME-8-labeled compartments. Coelomocytes are outlined. (Bar, 5 μm in all images.)
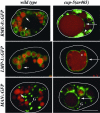
Fig. 4.
Rescue of the cup-5 coelomocyte endocytosis defect by expression of human homologues. Shown are epifluorescence images of cup-5(ar465); p_myo-3_::ssGFP worms expressing the indicated human genes in their coelomocytes. (Left) Low-magnification images showing whole worms (outlined in white). (Right) High-magnification images showing individual coelomocytes (outlined in white). GFP staining in rescued/WT coelomocytes is not obvious at this magnification in whole worms, whereas mutant coelomocytes are very bright. For comparison, we show a worm expressing human MCOLN1 with one of its coelomocytes that is not fully rescued (arrow). Due to their brightness, the mutant/nonrescued coelomocytes of control worms were photographed by using 1/10th the exposure time as the WT/rescued coelomocytes. (Bar, 5 μm in high-magnification images.)
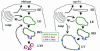
Fig. 5.
Model of CUP-5 function in coelomocytes. In WT, solutes and membrane are internalized and delivered to the early endosome (EE). Molecules from the endocytic and biosynthetic pathways are then delivered to late endosomes (LE). Lysosomal hydrolases and the solutes and internal vesicles destined for degradation are condensed in membrane structures budding off from these late endosomes. We refer to these structures as hybrid organelles (HO). The scission of the budding vesicles yields primary lysosomes (LYS), which, based on data from mammalian cells, can fuse with late endosomes or with each other. RME-8 (green) localizes to late endosomes. CUP-5 (red) and LMP-1 (blue) localize to sites of lysosome biogenesis on late endosomes. In cup-5 mutants, the maturation/scission of lysosomes budding from hybrid organelles is defective. These endosomes continue to receive membrane and solutes from the endocytic and biosynthetic pathways and hence increase in size. Due to their aberrant molecular composition, these large vacuoles (LV) contain less RME-8::GFP than late endosomes or hybrid organelles in WT cells. We do not know whether there is direct transport of biosynthetic material from the Golgi to these large vacuoles.
Similar articles
- Roles of CUP-5, the Caenorhabditis elegans orthologue of human TRPML1, in lysosome and gut granule biogenesis.
Campbell EM, Fares H. Campbell EM, et al. BMC Cell Biol. 2010 Jun 11;11:40. doi: 10.1186/1471-2121-11-40. BMC Cell Biol. 2010. PMID: 20540742 Free PMC article. - Suppression of the cup-5 mucolipidosis type IV-related lysosomal dysfunction by the inactivation of an ABC transporter in C. elegans.
Schaheen L, Patton G, Fares H. Schaheen L, et al. Development. 2006 Oct;133(19):3939-48. doi: 10.1242/dev.02575. Epub 2006 Aug 30. Development. 2006. PMID: 16943270 - Basis of lethality in C. elegans lacking CUP-5, the Mucolipidosis Type IV orthologue.
Schaheen L, Dang H, Fares H. Schaheen L, et al. Dev Biol. 2006 May 15;293(2):382-91. doi: 10.1016/j.ydbio.2006.02.008. Dev Biol. 2006. PMID: 16530747 - CUPpling calcium to lysosomal biogenesis.
Piper RC, Luzio JP. Piper RC, et al. Trends Cell Biol. 2004 Sep;14(9):471-3. doi: 10.1016/j.tcb.2004.07.010. Trends Cell Biol. 2004. PMID: 15350973 Review. - The molecular basis of mucolipidosis type IV.
Slaugenhaupt SA. Slaugenhaupt SA. Curr Mol Med. 2002 Aug;2(5):445-50. doi: 10.2174/1566524023362276. Curr Mol Med. 2002. PMID: 12125810 Review.
Cited by
- The lysosomal Ca2+ release channel TRPML1 regulates lysosome size by activating calmodulin.
Cao Q, Yang Y, Zhong XZ, Dong XP. Cao Q, et al. J Biol Chem. 2017 May 19;292(20):8424-8435. doi: 10.1074/jbc.M116.772160. Epub 2017 Mar 30. J Biol Chem. 2017. PMID: 28360104 Free PMC article. - The conserved SNARE SEC-22 localizes to late endosomes and negatively regulates RNA interference in Caenorhabditis elegans.
Zhao Y, Holmgren BT, Hinas A. Zhao Y, et al. RNA. 2017 Mar;23(3):297-307. doi: 10.1261/rna.058438.116. Epub 2016 Dec 14. RNA. 2017. PMID: 27974622 Free PMC article. - Regulators of Lysosome Function and Dynamics in Caenorhabditis elegans.
Gee K, Zamora D, Horm T, George L, Upchurch C, Randall J, Weaver C, Sanford C, Miller A, Hernandez S, Dang H, Fares H. Gee K, et al. G3 (Bethesda). 2017 Mar 10;7(3):991-1000. doi: 10.1534/g3.116.037515. G3 (Bethesda). 2017. PMID: 28122949 Free PMC article. - The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway.
Martina JA, Lelouvier B, Puertollano R. Martina JA, et al. Traffic. 2009 Aug;10(8):1143-56. doi: 10.1111/j.1600-0854.2009.00935.x. Epub 2009 Apr 29. Traffic. 2009. PMID: 19497048 Free PMC article. - A double TRPtych: six views of transient receptor potential channels in disease and health.
Cornell RA, Aarts M, Bautista D, García-Añoveros J, Kiselyov K, Liman ER. Cornell RA, et al. J Neurosci. 2008 Nov 12;28(46):11778-84. doi: 10.1523/JNEUROSCI.3929-08.2008. J Neurosci. 2008. PMID: 19005039 Free PMC article. Review.
References
- D'Hondt, K., Heese-Peck, A. & Riezman, H. (2000) Annu. Rev. Genet. 34, 255–295. - PubMed
- Sorkin, A. (2000) J. Cell Sci. 113, 4375–4376. - PubMed
- Lemmon, S. K. & Traub, L. M. (2000) Curr. Opin. Cell Biol. 12, 457–466. - PubMed
- Luzio, J. P., Poupon, V., Lindsay, M. R., Mullock, B. M., Piper, R. C. & Pryor, P. R. (2003) Mol. Membr. Biol. 20, 141–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous