TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes - PubMed (original) (raw)
TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes
Yufeng Peng et al. Proc Natl Acad Sci U S A. 2004.
Abstract
CD4+CD25+ regulatory T cells are essential in the protection from organ-specific autoimmune diseases. In the pancreas, they inhibit actions of autoreactive T cells and thereby prevent diabetes progression. The signals that control the generation, the maintenance, or the expansion of regulatory T cell pool in vivo remain poorly understood. Here we show that a transient pulse of transforming growth factor beta (TGF-beta) in the islets during the priming phase of diabetes is sufficient to inhibit disease onset by promoting the expansion of intraislet CD4+CD25+ T cell pool. Approximately 40-50% of intraislet CD4+ T cells expressed the CD25 marker and exhibited characteristics of regulatory T cells including small size, high level of intracellular CTLA-4, expression of Foxp3, and transfer of protection against diabetes. Results from in vivo incorporation of BrdUrd revealed that the generation of a high frequency of regulatory T cells in the islets is due to in situ expansion upon TGF-beta expression. Thus, these findings demonstrate a previously uncharacterized mechanism by which TGF-beta inhibits autoimmune diseases via regulation of the size of the CD4+CD25+ regulatory T cell pool in vivo.
Figures
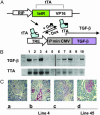
Fig. 1.
Regulated TGF-β expression in TTA/TGF-β NOD mice. (A) TTA regulatory system. Under the control of a rat insulin promoter (RIP), tetracycline-controlled transactivator (TTA) is expressed specifically in insulin-producing cells. In the presence of tetracycline or its analogue doxycycline (Dox), TTA (+Dox) is unable to bind the TRE and TGF-β transcription is inactive, whereas in the absence of doxycycline (–Dox) TTA binds TRE and induces TGF-β expression via the cytomegalovirus minimal promoter (P min CMV). DNA fragments RIP-TTA and TRE-TGF-β were coinjected to generate double transgenic mice in NOD background. (B) Regulated TGF-β transcription. NOD Transgenic mice were fed with normal or doxycycline-supplemented food. Total RNA was isolated from pancreatic tissue. The transcripts of TGF-β (Upper) and TTA (Lower) were amplified by using RT-PCR. Lanes 1 and 3, constitutively on for 5 weeks since birth; lanes 2 and 4, constitutively on for 5 weeks followed by 1-week turn-off; lane 5, transgene negative control; lane 6, constitutively off for 2 months since birth; lanes 7, 8, and 10, constitutively off for 2 month since birth followed by a turn-on for 4 days (lanes 7 and 10) or 1 week (lane 8); lane 9, spleen RNA controls. Each lane represents a different mouse. (C) Regulated TGF-β protein expression. Pancreatic tissues were isolated from line 4 (a_–_c) and line 45 (d), then fixed and stained for TGF-β protein (red, counterstained by hematoxylin/eosin). (a) Constitutively off for 8 weeks since birth. (b) Constitutively on for 5 weeks since birth. (c) Constitutively on for 5 weeks since birth followed by 1 week turn-off. (d) Constitutively on for 8 weeks since birth.
Fig. 2.
Transient expression of TGF-β inhibits diabetes. (A) Summary of the different groups (1–5) of transgenic mice with turning on and off period time of the expression of TGF-β. Development of diabetes was monitored in line 4 (B) and line 45 (C) transgenic mice during 60 weeks. Filled circle, transgene negative; open circle, TGF-β turned on and off (groups 2–5); filled triangle, TGF-β constitutively turned off (group 1).
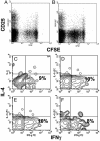
Fig. 3.
TGF-β expression does not affect intraislet antigen-presenting function or T cell differentiation profile. CFSE-labeled purified naïve BDC2.5 T cells were injected into 4-week-old transgenic mice (line 45) in which TGF-β was either turned off (A) or on (B) from birth to 4 weeks of age. Four days posttransfer, pancreatic lymph node cells were collected and lymphocytes were analyzed by FACS. Dot plots represent CFSE intensity on gated CD4+ T cells. The results are a representative of eight experiments. Similar results were obtained from line 4. Intraislet T cells were pooled from five transgenic mice (line 45) and stimulated with phorbol 12-myristate 13-acetate plus ionomycin for 6 h. Contour plots represent the distribution of IL-4-versus IFN-γ-expressing cells among gated CD4+ T cells isolated from mice with TGF-β constitutively off for 10 weeks since birth (C), with TGF-β off for 8 weeks since birth and turned on for the next 5 weeks (D), with TGF-β on for 8 weeks since birth (E), or with TGF-β on for 16 weeks since birth (F).
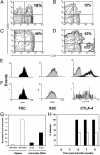
Fig. 4.
TGF-β induces high frequency of intraislet CD4+CD25+ regulatory T cells Intraislet T cells were pooled from five transgenic mice (line 45) and then stained for the expression of CD4 and CD25 markers. Contour plots represents results from mice with TGF-β constitutively off for 10 weeks since birth (A), TGF-β off for 8 weeks since birth and turned on for the next 5 weeks (B), TGF-β on for 8 weeks since birth (C), or TGF-β on for 16 weeks since birth (D). Gated CD4+CD25+ T cells from pancreatic lymph nodes (E) or islets (F) isolated from transgenic line 45 with TGF-β on for 8 weeks since birth were compared for intracellular expression of CTLA-4 as well as cell size (FSC) and cell granulosity (SSC). (G) Intraislet CD4+ T cells were purified from transgenic line 45 with TGF-β on for 8 weeks since birth (Tg pos) or from transgenic negative mice (Tg neg), and levels of mRNA of Foxp3 expression were determined by real-time quantitative PCR (filled bars). Splenic CD4+CD25+ and CD4+CD25– T cells from NOD mice were used as controls (open bars). (H) Intraislet T cells were purified from transgenic line 45 with TGF-β on for 8 weeks since birth, and 105 cells were transferred to NOD-SCID recipient mice together with 20 × 106 splenic cells from diabetic NOD mice (open bars). As control, 20 × 106 splenic cells from diabetic NOD mice were transferred alone to recipient mice (filled bars). Diabetes development was monitored for 10 weeks posttransfer.
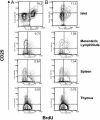
Fig. 5.
TGF-β induces proliferation of intraislet CD4+CD25+ T cells. Control NOD mice (B) and transgenic line 45 with TGF-β on for 8 weeks since birth (A) were injected for 4 consecutive days with 1 mg of BrdUrd. Lymphocytes were isolated from islets, mesenteric lymph nodes, spleen, and thymus, and stained with anti-CD4 and anti-CD25 and anti-BrdUrd antibodies. Contour plots show the distribution of BrdUrd versus CD25 expression on gated CD4+ T cells. The results are representative of four pooled mice.
Similar articles
- CD4+CD25+ regulatory T cells generated in response to insulin B:9-23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells.
Mukherjee R, Chaturvedi P, Qin HY, Singh B. Mukherjee R, et al. J Autoimmun. 2003 Nov;21(3):221-37. doi: 10.1016/s0896-8411(03)00114-8. J Autoimmun. 2003. PMID: 14599847 - CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes.
Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. Green EA, et al. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10878-83. doi: 10.1073/pnas.1834400100. Epub 2003 Aug 29. Proc Natl Acad Sci U S A. 2003. PMID: 12949259 Free PMC article. - Requirement of CD28 signaling in homeostasis/survival of TGF-beta converted CD4+CD25+ Tregs from thymic CD4+CD25- single positive T cells.
Liu Y, Amarnath S, Chen W. Liu Y, et al. Transplantation. 2006 Oct 15;82(7):953-64. doi: 10.1097/01.tp.0000232330.46688.37. Transplantation. 2006. PMID: 17038912 - Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Chen W, et al. J Exp Med. 2003 Dec 15;198(12):1875-86. doi: 10.1084/jem.20030152. J Exp Med. 2003. PMID: 14676299 Free PMC article. - Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease.
Horwitz DA, Zheng SG, Gray JD, Wang JH, Ohtsuka K, Yamagiwa S. Horwitz DA, et al. Semin Immunol. 2004 Apr;16(2):135-43. doi: 10.1016/j.smim.2003.12.009. Semin Immunol. 2004. PMID: 15036237 Review.
Cited by
- Control of autoimmune inflammation by celastrol, a natural triterpenoid.
Venkatesha SH, Dudics S, Astry B, Moudgil KD. Venkatesha SH, et al. Pathog Dis. 2016 Aug;74(6):ftw059. doi: 10.1093/femspd/ftw059. Epub 2016 Jul 11. Pathog Dis. 2016. PMID: 27405485 Free PMC article. Review. - Targeting Stem Cell-Derived Tissue-Associated Regulatory T Cells for Type 1 Diabetes Immunotherapy.
Haque M, Das JK, Xiong X, Song J. Haque M, et al. Curr Diab Rep. 2019 Aug 30;19(10):89. doi: 10.1007/s11892-019-1213-7. Curr Diab Rep. 2019. PMID: 31471667 Free PMC article. Review. - Remission of collagen-induced arthritis is associated with high levels of transforming growth factor-beta expression in the joint.
Marinova-Mutafchieva L, Gabay C, Funa K, Williams RO. Marinova-Mutafchieva L, et al. Clin Exp Immunol. 2006 Nov;146(2):287-93. doi: 10.1111/j.1365-2249.2006.03204.x. Clin Exp Immunol. 2006. PMID: 17034581 Free PMC article. - Is transplantation tolerable?
Strom TB. Strom TB. J Clin Invest. 2004 Jun;113(12):1681-3. doi: 10.1172/JCI22153. J Clin Invest. 2004. PMID: 15199402 Free PMC article. - Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice.
Filippi CM, Estes EA, Oldham JE, von Herrath MG. Filippi CM, et al. J Clin Invest. 2009 Jun;119(6):1515-23. doi: 10.1172/JCI38503. Epub 2009 May 26. J Clin Invest. 2009. PMID: 19478458 Free PMC article.
References
- Bach, J. F. & Chatenoud, L. (2001) Annu. Rev. Immunol. 19, 131–161. - PubMed
- Gershon, R. K. (1975) Transplant Rev. 26, 170–185. - PubMed
- Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151–1164. - PubMed
- Groux, H., O'Garra, A., Bigler, M., Rouleau, M., Antonenko, S., de Vries, J. E. & Roncarolo, M. G. (1997) Nature 389, 737–742. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials