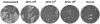Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge - PubMed (original) (raw)
Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge
Linda S Wyatt et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Modified vaccinia virus Ankara (MVA), developed >30 years ago as a highly attenuated candidate smallpox vaccine, was recloned from a 1974 passage and evaluated for safety and immunogenicity. Replication of MVA is impaired in most mammalian cells, and we found that mice with severe combined immunodeficiency disease remained healthy when inoculated with MVA at 1,000 times the lethal dose of vaccinia virus derived from the licensed Dryvax vaccine seed. In BALB/c mice inoculated intramuscularly with MVA, virus-specific CD8+ T cells and antibodies to purified virions and membrane protein components of the intracellular and extracellular infectious forms of vaccinia virus were induced in a dose-dependent manner. After one or two inoculations of MVA, the T cell numbers and antibody titers equaled or exceeded those induced by percutaneous injection of Dryvax. Antibodies induced by MVA and Dryvax were neutralizing and inhibited virus spread in cultured cells. Furthermore, vaccinated mice were protected against lethal intranasal challenge with a pathogenic vaccinia virus. B cell-deficient mice unable to generate antibodies and beta2-microglobulin-deficient mice unable to express MHC class I molecules for a CD8+ T cell response were also protectively vaccinated by MVA. In contrast, mice with decreased CD4 or MHC class II expression and double-knockout mice deficient in MHC class I- and II-restricted activities were poorly protected or unprotected. This study confirmed the safety of MVA and demonstrated that the overlapping immune responses protected normal and partially immune-deficient animals, an encouraging result for this candidate attenuated smallpox vaccine.
Figures
Fig. 1.
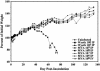
Safety of MVA. SCID mice were uninfected, infected i.p. with MVA (106 to 109 pfu) or Wyeth strain of vaccinia virus (105 to 106 pfu), or infected i.v. with 108 pfu of MVA. Mice were weighed individually, and the averages were plotted. †, died naturally or were killed because of 30% weight loss. Only mice inoculated with 106 pfu of the Wyeth strain of vaccinia virus showed persistent weight loss, disease, and death.
Fig. 2.
Anti-comet test. After BS-C-1 cells were infected with the IHD strain of vaccinia virus, pooled serum diluted 1:50 from mice immunized twice with MVA (106 to 108 pfu) or once with Dryvax was added to the liquid overlay medium. After 48 h, the monolayers were stained with crystal violet.
Fig. 3.
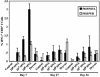
CD8+ T cell responses. BALB/c mice were unimmunized (controls) or immunized i.m. with MVA at 106, 107, or 108 pfu or percutaneously with Dryvax on day 0. On the indicated days, spleens were removed and mixed with P815 cells that had been stimulated overnight with MVA or WR. The percentage of vaccinia virus-specific IFN-γ expressing CD3+ CD8+ cells were determined for four animals in each group, and the values were plotted with standard deviations. On day 30, mice were challenged i.n. with 106 pfu of vaccinia virus WR.
Fig. 4.
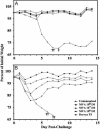
Protective immunization of mice. (A) Groups of four BALB/c mice were inoculated i.m. with MVA (106 to 108 pfu) or percutaneously with Dryvax and challenged 4 weeks later by i.n. inoculation of 106 pfu of vaccinia virus WR. (B) BALB/c mice vaccinated with MVA as in A were revaccinated after 4 weeks and challenged 3 weeks after that. Dryvax-vaccinated mice were challenged at 7 weeks after the single vaccination. Mice were weighed individually and averages were plotted. †, Died naturally or were killed because of 30% weight loss. The mice used here were the same as those in Table 1.
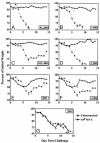
Fig. 5.
Protective immunization of immune-deficient mice. Mice (n = 4) were vaccinated once i.m. with 108 pfu of MVA and challenged 3 weeks later with 106 pfu of vaccinia virus WR by the i.n. route. (A) BALB/c mice. (B) B cell-deficient mice. (C) C57BL/6 mice. (D) β2-microglobulin-deficient mice. (E) CD4-deficient mice. (F) MHC class II-deficient mice. (G) Double-knockout mice deficient in MHC class I and II. Inset numbers represent the IMV reciprocal endpoint ELISA titers at 3 weeks.
Similar articles
- Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model.
Meseda CA, Garcia AD, Kumar A, Mayer AE, Manischewitz J, King LR, Golding H, Merchlinsky M, Weir JP. Meseda CA, et al. Virology. 2005 Sep 1;339(2):164-75. doi: 10.1016/j.virol.2005.06.002. Virology. 2005. PMID: 15993917 - Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4.
McCurdy LH, Rutigliano JA, Johnson TR, Chen M, Graham BS. McCurdy LH, et al. J Virol. 2004 Nov;78(22):12471-9. doi: 10.1128/JVI.78.22.12471-12479.2004. J Virol. 2004. PMID: 15507634 Free PMC article. - Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines.
Drexler I, Staib C, Kastenmuller W, Stevanović S, Schmidt B, Lemonnier FA, Rammensee HG, Busch DH, Bernhard H, Erfle V, Sutter G. Drexler I, et al. Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):217-22. doi: 10.1073/pnas.262668999. Epub 2002 Dec 23. Proc Natl Acad Sci U S A. 2003. PMID: 12518065 Free PMC article. - Monitoring of human immunological responses to vaccinia virus.
Harrop R, Ryan MG, Golding H, Redchenko I, Carroll MW. Harrop R, et al. Methods Mol Biol. 2004;269:243-66. doi: 10.1385/1-59259-789-0:243. Methods Mol Biol. 2004. PMID: 15114020 Review. - Clinical development of Modified Vaccinia virus Ankara vaccines.
Gilbert SC. Gilbert SC. Vaccine. 2013 Sep 6;31(39):4241-6. doi: 10.1016/j.vaccine.2013.03.020. Epub 2013 Mar 21. Vaccine. 2013. PMID: 23523410 Review.
Cited by
- Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice.
Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Davies DH, et al. J Virol. 2005 Sep;79(18):11724-33. doi: 10.1128/JVI.79.18.11724-11733.2005. J Virol. 2005. PMID: 16140750 Free PMC article. - Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity.
Zhang N, Cheng X, Zhu Y, Mo O, Yu H, Zhu L, Zhang J, Kuang L, Gao Y, Cao R, Liang X, Wang H, Li H, Li S, Zhong W, Li X, Li X, Hao P. Zhang N, et al. Sci China Life Sci. 2023 Oct;66(10):2329-2341. doi: 10.1007/s11427-023-2378-x. Epub 2023 Jun 1. Sci China Life Sci. 2023. PMID: 37300753 Free PMC article. - Production of Modified Vaccinia Ankara Virus by Intensified Cell Cultures: A Comparison of Platform Technologies for Viral Vector Production.
Gränicher G, Tapia F, Behrendt I, Jordan I, Genzel Y, Reichl U. Gränicher G, et al. Biotechnol J. 2021 Jan;16(1):e2000024. doi: 10.1002/biot.202000024. Epub 2020 Sep 8. Biotechnol J. 2021. PMID: 32762152 Free PMC article. - MVA recombinants expressing the fusion and hemagglutinin genes of PPRV protects goats against virulent challenge.
Chandran D, Reddy KB, Vijayan SP, Sugumar P, Rani GS, Kumar PS, Rajendra L, Srinivasan VA. Chandran D, et al. Indian J Microbiol. 2010 Sep;50(3):266-74. doi: 10.1007/s12088-010-0026-9. Epub 2010 Mar 16. Indian J Microbiol. 2010. PMID: 23100840 Free PMC article. - Critical role of perforin-dependent CD8+ T cell immunity for rapid protective vaccination in a murine model for human smallpox.
Kremer M, Suezer Y, Volz A, Frenz T, Majzoub M, Hanschmann KM, Lehmann MH, Kalinke U, Sutter G. Kremer M, et al. PLoS Pathog. 2012;8(3):e1002557. doi: 10.1371/journal.ppat.1002557. Epub 2012 Mar 1. PLoS Pathog. 2012. PMID: 22396645 Free PMC article.
References
- Henderson, D. A. (1999) Science 283, 1279-1282. - PubMed
- Fenner, F., Henderson, D. A., Arita, I., Jezek, Z. & Ladnyi, I. D. (1988) Smallpox and Its Eradication (World Health Organization, Geneva).
- Fulginiti, V. A., Papier, A., Lane, J. M., Neff, J. M. & Henderson, D. A. (2003) Clin. Infect. Dis. 37, 251-271. - PubMed
- Mayr, A., Hochstein-Mintzel, V. & Stickl, H. (1975) Infection 3, 6-14.
- Stickl, H., Hochstein-Mintzel, V., Mayr, A., Huber, H. C., Schäfer, H. & Holzner, A. (1974) Dtsch. Med. Wochenschr. 99, 2386-2392. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials