Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C - PubMed (original) (raw)
Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C
Michael A Sutton et al. J Neurosci. 2004.
Abstract
Recent studies of long-term synaptic plasticity and long-term memory have demonstrated that the same functional endpoint, such as long-term potentiation, can be induced through distinct signaling pathways engaged by different patterns of stimulation. A critical question raised by these studies is whether different induction pathways either converge onto a common molecular mechanism or engage different molecular cascades for the maintenance of long-term plasticity. We directly examined this issue in the context of memory for sensitization in the marine mollusk Aplysia. In this system, training with a single tail shock normally induces short-term memory (<30 min) for sensitization of tail-elicited siphon withdrawal, whereas repeated spaced shocks induce both intermediate-term memory (ITM) (>90 min) and long-term memory (>24 hr). We now show that a single tail shock can also induce ITM that is expressed selectively at the trained site (site-specific ITM). Although phenotypically similar to the form of ITM induced by repeated trials, the mechanisms by which site-specific ITM is induced and maintained are distinct. Unlike repeated-trial ITM, site-specific ITM requires neither protein synthesis nor PKA activity for induction or maintenance. Rather, the induction of site-specific ITM requires calpain-dependent proteolysis of activated PKC, yielding a persistently active PKC catalytic fragment (PKM) that also serves to maintain the memory in the intermediateterm temporal domain. Thus, two unique forms of ITM that have different induction requirements also use distinct molecular mechanisms for their maintenance.
Figures
Figure 1.
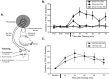
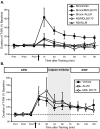
A single shock induces site-specific intermediate-term memory in a reduced preparation. A, Illustration of the reduced preparation depicting the sites on the tail used for training and testing. All pharmacological treatments were restricted to the ring ganglia (indicated by shading). Independent groups of animals received a single tail shock to the test site itself (shock test; n = 7), a different tail site ipsilateral to the test site (shock ipsilateral to test site; n = 6), or to a site contralateral to the test site (shock contralateral to test site; n = 4). B, Mean ± SEM normalized duration of T-SW in the three groups (Shock Test, Shock Ipsi, and Shock Contra) over time before and at various times after training. A single shock induces short-term memory (<30 min) at nonshocked sites and intermediate-term memory (> 90 min) at the shocked site. Pre1–Pre3, Pretests 1–3. In this and subsequent figures, the horizontal dashed line indicates baseline, and the arrow indicates approximate time of training. C, Comparison of intermediate-term memory for sensitization of T-SW in the reduced preparation induced either by repeated tail shocks to an ipsilateral site distinct from that used for testing (5×S nontest site; n = 7) or by a single shock to the test site itself (1×S test site; data replotted from part B). With each pattern of experience, memory for sensitization persists for >75 min after training and is of similar magnitude. Error bars represent SEM.
Figure 2.
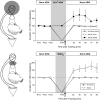
Plasticity in the ring ganglia is necessary and sufficient for site-specific ITM. Top, The CNS was inactivated by perfusion of the ring ganglia with modified ASW containing 3× Mg2+ and no added Ca2+. The mean ± SEM duration of T-SW in preparations that were trained with a single shock to the test site with (n = 4) or without (n = 6) CNS inactivation are shown. Recovery from CNS inactivation was assessed in nontrained preparations perfused with 3× Mg2+ and no Ca2+ ASW in parallel (n = 3). Control preparations treated with normal ASW (Norm ASW) demonstrated robust site-specific ITM for sensitization at the shocked site; this effect was completely blocked by CNS inactivation. After washout, T-SW recovered completely to baseline levels in nontrained preparations. Bottom, Inactivation of the abdominal ganglion during training was achieved by isolating short segments (∼1–2 mm) of both pleural–abdominal nerves in a separate subchamber and substituting ASW with isotonic MgCl2 to block action potential conduction. After a complete bath exchange of the subchamber, a fourth pretest of T-SW by an independent observer was taken to verify functional blockade. After this test, the MgCl2 remained in the subchamber for 5 min before the application of a single tail shock to the test site and for an additional 5 min period after training, after which perfusion of the subchamber with ASW resumed. The mean ± SEM duration of T-SW in preparations that received either a single shock to the test site (n = 5) or no shock (n = 3) with the abdominal ganglion inactivated are shown. Inactivation of the abdominal ganglion had no effect on the induction of site-specific ITM. Pre1–Pre4, Pretests 1–4.
Figure 3.
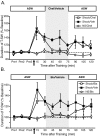
Site-specific ITM has a unique molecular mechanism. A, Mean ± SEM normalized duration of T-SW over time in preparations treated with or without the protein synthesis inhibitor emetine (100 μ
m
) throughout the experiment (n = 6 per group). B, Mean ± SEM normalized duration of T-SW over time in preparations treated with the PKA inhibitor KT5720 (10 μ
m
) or vehicle (1% DMSO) for 30 min before and during training with a single shock to the test site (n = 5 per group). KT5720–vehicle was added to the ring ganglia subchamber after completion of the pretests and was washed out immediately after training. C, Mean ± SEM normalized duration of T-SW over time in preparations treated with 10 μ
m
KT5720 (n = 6) or vehicle (n = 7) beginning 30 min after training with a single shock to the test site. KT5720–vehicle (KT/Vehicle) was washed out immediately after the 75 min post-test. Drug–vehicle application is indicated by shading. Pre1–Pre3, Pretests 1–3.
Figure 4.
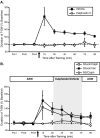
Site-specific ITM is maintained by a persistent activation of PKC. A, Mean ± SEM normalized duration of T-SW over time in preparations treated with the PKC inhibitor chelerythrine (Chel) (50 μ
m
; n = 6) or vehicle (Veh) (distilled water; n = 7) beginning 30 min after training with a single shock to the test site. Nontrained preparations treated with chelerythrine over the same time period (n = 3) were examined in parallel. B, Mean ± SEM normalized duration of T-SW over time in preparations treated with the PKC inhibitor bisindolylmaleimide I (Bis) (10 μ
m
; n = 6) or vehicle (0.5% DMSO; n = 6) beginning 30 min after training with a single shock to the test site. Nontrained preparations treated with bisindolylmaleimide I over the same time period (n = 3) were examined in parallel. In both experiments, drug–vehicle was washed out immediately after the 75 min post-test. Drug–vehicle application is indicated by shading. Pre1–Pre3, Pretests 1–3.
Figure 5.
Calphostin C, a PKC inhibitor interacting with the regulatory domain, blocks the induction but not the maintenance of site-specific ITM. A, Mean ± SEM normalized duration of T-SW over time in preparations treated with the PKC inhibitor calphostin C (5μ
m
) or vehicle (1% DMSO) for 45 min before and during training with a single shock to the test site (n = 6 per group). Calphostin–vehicle was added to the ring ganglia subchamber after completion of the pretests and was washed out immediately after training. Calphostin C completely blocked the induction of site-specific ITM. B, Mean ± SEM normalized duration of T-SW over time in preparations treated with 5 μ
m
calphostin C (Calph; n = 6) or vehicle (Veh; n = 8) beginning 30 min after training with a single shock to the test site. Nontrained preparations treated with calphostin C over the same time period (n = 3) were examined in parallel. Calphostin–vehicle was washed out immediately after the 75 min post-test. Drug–vehicle application is indicated by shading. The maintenance of site-specific ITM was not affected by calphostin C. Note that the calphostin C incubation time in this experiment is identical to that in A. Pre1–Pre3, Pretests 1–3.
Figure 6.
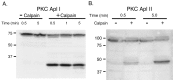
Calpain proteolyses endogenous Aplysia PKC. Western blots of Aplysia CNS probed with antibodies against the catalytic domain of the Ca2+-dependent PKC isoform Apl I (A) and the Ca2+-independent PKC isoform Apl II (B) are shown. Whole CNS homogenate (pooled from 2 to 4 animals) was incubated either with homogenization buffer alone (–Calpain) or with homogenization buffer containing m-calpain (+Calpain; 6 U per CNS) for the times indicated. Equal amounts of the homogenate were loaded in each lane (∼0.5 CNS per lane). Approximate molecular weights (in kilodaltons) are indicated on the left. The addition of calpain generates a catalytic fragment of Apl I (∼30 kDa) and Apl II (∼45 kDa). The examples shown are representative of four independent experiments.
Figure 7.
Activity of the Ca2+-activated protease calpain is required for the induction but not the maintenance of site-specific ITM. A, Mean ± SEM normalized duration of T-SW over time in preparations treated with the calpain inhibitors ALLM (100μ
m
; n = 6), MDL28170 (100 μ
m
; n = 6), or vehicle (Veh) (1% DMSO; n = 10) for 45 min before and during training with a single shock to the test site. Nontrained preparations treated with ALLM (n = 3) or MDL28170 (n = 2) were examined in parallel. Drugs–vehicle were added to the ring ganglia subchamber after completion of the pretests and were washed out immediately after training. Both calpain inhibitors blocked the induction of site-specific ITM. B, Mean ± SEM normalized duration of T-SW over time in preparations treated with 100 μ
m
ALLM (n = 5), 100 μ
m
MDL28170 (n = 5), or vehicle (n = 6) beginning 30 min after training with a single shock to the test site. Drugs–vehicle were washed out immediately after the 75 min post-test. Drug–vehicle application is indicated by shading. The maintenance of site-specific ITM was not affected by either calpain inhibitor. Note that the incubation time for each calpain inhibitor in this experiment is identical to that in A. Pre1–Pre3, Pretests 1–3.
Figure 8.
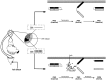
A model for the induction and maintenance of site-specific ITM. A schematic representation of the proposed molecular events related to PKC activation in tail SNs (or possibly interneurons activated by these SNs) after tail shock is shown. Under basal conditions, PKC is retained in the cytosol, and its catalytic activity is inhibited by the N-terminal regulatory domain (R) that acts as a pseudosubstrate for the C-terminal catalytic domain (C). A single tail shock, regardless of location, will elicit 5-HT release (shaded area) that is distributed across the entire population of SNs and their synapses (Marinesco and Carew, 2002). 5-HT, acting through phospholipase C-coupled receptors (Li et al., 1995), stimulates translocation of PKC to the membrane, inducing a conformational change that relieves the autoinhibition of PKC activity by the regulatory domain. In SNs with receptive fields that lie outside the region of the tail receiving shock (open circles and top), 5-HT-induced PKC activation will be transient, enduring only as long as the short-term modulatory effects of 5-HT on second messengers persist. Conversely, the SNs innervating the shocked site will be activated (Walters et al., 1983a,b) coincident with 5-HT exposure (filled circles and bottom). SN activation increases intracellular Ca2+ influx, stimulating the Ca2+-activated protease calpain. Calpain, in turn, proteolyses activated PKC, generating persistently active PKMs. Thus, the induction of site-specific sensitization entails the proteolytic processing of activated PKC, the product of which (PKM) serves to maintain the memory in the intermediate-term temporal domain.
Similar articles
- Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia.
Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ. Sharma SK, et al. J Neurosci. 2003 May 1;23(9):3899-907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. J Neurosci. 2003. PMID: 12736359 Free PMC article. - Massed training-induced intermediate-term operant memory in aplysia requires protein synthesis and multiple persistent kinase cascades.
Michel M, Green CL, Gardner JS, Organ CL, Lyons LC. Michel M, et al. J Neurosci. 2012 Mar 28;32(13):4581-91. doi: 10.1523/JNEUROSCI.6264-11.2012. J Neurosci. 2012. PMID: 22457504 Free PMC article. - Molecular mechanisms underlying a unique intermediate phase of memory in aplysia.
Sutton MA, Masters SE, Bagnall MW, Carew TJ. Sutton MA, et al. Neuron. 2001 Jul 19;31(1):143-54. doi: 10.1016/s0896-6273(01)00342-7. Neuron. 2001. PMID: 11498057 - Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.
Rahn EJ, Guzman-Karlsson MC, David Sweatt J. Rahn EJ, et al. Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review. - Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia.
Bailey CH, Kandel ER. Bailey CH, et al. Prog Brain Res. 2008;169:179-98. doi: 10.1016/S0079-6123(07)00010-6. Prog Brain Res. 2008. PMID: 18394474 Review.
Cited by
- Nonassociative learning in invertebrates.
Byrne JH, Hawkins RD. Byrne JH, et al. Cold Spring Harb Perspect Biol. 2015 Feb 26;7(5):a021675. doi: 10.1101/cshperspect.a021675. Cold Spring Harb Perspect Biol. 2015. PMID: 25722464 Free PMC article. Review. - Cycloheximide impairs and enhances memory depending on dose and footshock intensity.
Gold PE, Wrenn SM. Gold PE, et al. Behav Brain Res. 2012 Aug 1;233(2):293-7. doi: 10.1016/j.bbr.2012.05.010. Epub 2012 May 17. Behav Brain Res. 2012. PMID: 22610049 Free PMC article. - Possible novel features of synaptic regulation during long-term facilitation in Aplysia.
Jin I, Kassabov S, Kandel ER, Hawkins RD. Jin I, et al. Learn Mem. 2021 Jun 15;28(7):218-227. doi: 10.1101/lm.053124.120. Print 2021 Jul. Learn Mem. 2021. PMID: 34131053 Free PMC article. - Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity.
Hu JY, Chen Y, Schacher S. Hu JY, et al. J Neurosci. 2007 Aug 15;27(33):8927-39. doi: 10.1523/JNEUROSCI.2322-07.2007. J Neurosci. 2007. PMID: 17699674 Free PMC article. - Unique ionotropic receptors for D-aspartate are a target for serotonin-induced synaptic plasticity in Aplysia californica.
Carlson SL, Fieber LA. Carlson SL, et al. Comp Biochem Physiol C Toxicol Pharmacol. 2012 Jan;155(1):151-9. doi: 10.1016/j.cbpc.2011.04.001. Epub 2011 Apr 9. Comp Biochem Physiol C Toxicol Pharmacol. 2012. PMID: 21497673 Free PMC article.
References
- Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER (2000) A novel function for serotonin-mediated short-term facilitation in Aplysia: conversion of a transient, cell-wide homosynaptic hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci USA 97: 11581–11586. - PMC - PubMed
- Berman DE, Dudai Y (2001) Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291: 2417–2419. - PubMed
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER (1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237. - PubMed
- Castellucci VF, Blumenfeld H, Goelet P, Kandel ER (1989) Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol 20: 1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous