Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling - PubMed (original) (raw)
. 2004 Apr 27;101(17):6553-8.
doi: 10.1073/pnas.0401496101. Epub 2004 Apr 7.
Douglas J Hilton, Donald Metcalf, Jennifer L Antonchuk, Craig D Hyland, Sandra L Mifsud, Ladina Di Rago, Adrienne A Hilton, Tracy A Willson, Andrew W Roberts, Robert G Ramsay, Nicos A Nicola, Warren S Alexander
Affiliations
- PMID: 15071178
- PMCID: PMC404083
- DOI: 10.1073/pnas.0401496101
Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling
Marina R Carpinelli et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Genetic screens in lower organisms, particularly those that identify modifiers of preexisting genetic defects, have been used successfully to order components of complex signaling pathways. To date, similar suppressor screens have not been used in vertebrates. To define the molecular pathways regulating platelet production, we have executed a large-scale modifier screen with genetically thrombocytopenic Mpl(-/-) mice by using N-ethyl-N-nitrosourea mutagenesis. Here we show that mutations in the c-Myb gene cause a myeloproliferative syndrome and supraphysiological expansion of megakaryocyte and platelet production in the absence of thrombopoietin signaling. This screen demonstrates the utility of large-scale N-ethyl-N-nitrosourea mutagenesis suppressor screens in mice for the simultaneous discovery and in vivo validation of targets for therapeutic discovery in diseases for which mouse models are available.
Figures
Fig. 1.
Identification of ENU-mutant mice with ameliorated thrombocytopenia. Peripheral blood platelet counts at 7 weeks of age for 100 Mpl+/+mice (a), 783 Mpl_-/_- mice (b), 1,575 G1 Mpl_-/_- mice harboring random ENU-induced mutations (c), the Plt3 G1 mouse (d), the G2 progeny derived from mating the G1 Plt3 mouse to Mpl_-/_- mice (e), the progeny derived from crossing heterozygous _Plt3/_+ Mpl_-/_- mice (f), the G1 Plt4 mouse (g), the G2 progeny derived from mating the G1 Plt4 mouse to Mpl_-/_- mice (h), the progeny derived from intercrossing heterozygous _Plt4/_+ Mpl_-/_- mice (i), the progeny derived from mating homozygous Plt4/Plt4 Mpl_-/_- mice to Mpl_-/_- mice (j), and the progeny derived from intercrossing homozygous Plt4/Plt4 Mpl_-/_- mice (k).
Fig. 2.
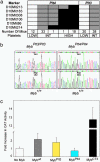
Plt3 and Plt4 are tightly linked on chromosome 10 and are alleles of c-Myb. (a) To map Plt4, a (C57BL/6 × 129Sv)F2 generation of 65 mice was produced, bled at 7 weeks of age, and categorized as having low platelets (<150 × 106/ml) characteristic of +/+ _Mpl_-_/_- mice, moderate numbers of platelets (150 × 106 to 2,000 × 106/ml) characteristic of _Plt4/_+ _Mpl_-_/_- mice, or extremely high platelets (>2,000 × 106/ml) characteristic of Plt4/Plt4 Mpl_-/_- mice. Animals were then genotyped, and markers found to be homozygous 129/Sv are shown in white, markers that were heterozygous are shown in gray, and markers homozygous C57BL/6 are shown in black. The number of animals with each haplotype is shown below. The Plt4 mutation was localized to between D10Mit213 and D10Mit 214. To confirm Plt3 was located close to Plt4, we produced 68 (C57BL/6 × 129Sv)N2 generation mice, measured platelet numbers in these animals, and genotyped them by using the markers most closely linked to Plt4. (Right) Correlation between genotype and phenotype for markers at the centromeric region of chromosome 10. (b) Sequence of PCR-amplified exons and intron boundaries of the c-Myb gene showing a single A to T transversion in Plt3 and Plt4, resulting in an Asp to Val substitution at positions 152 and 382, respectively. Representative traces are shown; a total of three Plt3/Pl3 Mpl_-/_-, three Plt3/+ Mpl_-/_- mice, three Plt4/Plt4 Mpl_-/_- mice, three Plt4/+ Mpl_-/- mice, three +/+ Mpl_-/- mice, and three +/+ Mpl+/+ mice were analyzed. (c) The transactivation activity of wild-type c-Myb, Plt3 and Plt4 mutant c-Myb, and a constitutively activated truncation of c-Myb (CT3; ref. 26) were compared by measuring production of chloramphenicol acetyltransferase from a c-Myb responsive promoter (28). The activity of both Plt3 and Plt4 c-Myb was significantly lower than wild type; (P = 0.017 and P = 0.0003, respectively; n = 9).
Fig. 3.
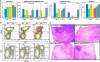
Mutation of c-Myb results in an elevation in progenitor cells, megakaryocytes, and platelets independent of Mpl. (a) The numbers of colony-forming units (spleen), a measure of multipotential progenitor cells, in the bone marrow (Far Left), clonogenic megakaryocyte progenitor cells (Left), megakaryocytes (Right) and platelets (Far Right)in c-Myb+/+ (+/+), c-Myb Plt4/+ (4/+), c-Myb Plt4/Plt4 (4/4), c-Myb Plt3/+ (3/+), and c-Myb Plt3/Plt3 (3/3) mutants on a Mpl_-/- or Mpl+/+ background are shown. Assays were performed as described in Materials and Methods with the error bars representing the SD from the mean of data from 3–7 [colony-forming units (spleen)], 2–6 (progenitor cell data), 2–7 (megakaryocyte data), and 4–50 (platelet data) mice. (b) Flow cytometric analysis of B lymphoid cells in the spleen and erythroid cells in the bone marrow of Plt4 mutant mice showing marked reductions in preB (B220+IgM-) and B (B220+IgM+) lymphocytes and accumulation of more immature erythroid cells (CD71hiTer119med and CD71hiTer119hi; ref. 31) in Mpl_-/- c-MybPlt4/Plt4 mice. (c) Histological sections of spleens from Mpl+/+ c-Myb+/+, Mpl -_/- c-MybPlt3/Plt3, Mpl_-/- c-MybPlt4/Plt4, and Mpl+/+ c-MybPlt4/Plt4 mice. Note the poor development of lymphoid follicles and expanded red pulp displaying reduced cellularity and disrupted architecture.
Comment in
- Modifier screens in the mouse: time to move forward with reverse genetics.
Curtis DJ. Curtis DJ. Proc Natl Acad Sci U S A. 2004 May 11;101(19):7209-10. doi: 10.1073/pnas.0401969101. Epub 2004 May 5. Proc Natl Acad Sci U S A. 2004. PMID: 15128944 Free PMC article. No abstract available.
Similar articles
- IL-3 does not contribute to platelet production in c-Mpl-deficient mice.
Chen Q, Solar G, Eaton DL, de Sauvage FJ. Chen Q, et al. Stem Cells. 1998;16 Suppl 2:31-6. doi: 10.1002/stem.5530160706. Stem Cells. 1998. PMID: 11012175 - c-mpl, the thrombopoietin receptor.
Vainchenker W, Methia N, Debili N, Titeux M, Wendling F. Vainchenker W, et al. Thromb Haemost. 1995 Jul;74(1):526-8. Thromb Haemost. 1995. PMID: 8578519 Review. - Studies of the c-Mpl thrombopoietin receptor through gene disruption and activation.
Alexander WS, Roberts AW, Maurer AB, Nicola NA, Dunn AR, Metcalf D. Alexander WS, et al. Stem Cells. 1996;14 Suppl 1:124-32. doi: 10.1002/stem.5530140716. Stem Cells. 1996. PMID: 11012212 Review. - Cloning and functional characterization of a novel c-mpl variant expressed in human CD34 cells and platelets.
Li J, Sabath DF, Kuter DJ. Li J, et al. Cytokine. 2000 Jul;12(7):835-44. doi: 10.1006/cyto.1999.0654. Cytokine. 2000. PMID: 10880227 - Dissection of c-Mpl and thrombopoietin function: studies of knockout mice and receptor signal transduction.
Gurney AL, de Sauvage FJ. Gurney AL, et al. Stem Cells. 1996;14 Suppl 1:116-23. doi: 10.1002/stem.5530140715. Stem Cells. 1996. PMID: 11012211 Review.
Cited by
- Mpl Baltimore: a thrombopoietin receptor polymorphism associated with thrombocytosis.
Moliterno AR, Williams DM, Gutierrez-Alamillo LI, Salvatori R, Ingersoll RG, Spivak JL. Moliterno AR, et al. Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11444-7. doi: 10.1073/pnas.0404241101. Epub 2004 Jul 21. Proc Natl Acad Sci U S A. 2004. PMID: 15269348 Free PMC article. - Dll1 haploinsufficiency in adult mice leads to a complex phenotype affecting metabolic and immunological processes.
Rubio-Aliaga I, Przemeck GK, Fuchs H, Gailus-Durner V, Adler T, Hans W, Horsch M, Rathkolb B, Rozman J, Schrewe A, Wagner S, Hoelter SM, Becker L, Klopstock T, Wurst W, Wolf E, Klingenspor M, Ivandic BT, Busch DH, Beckers J, Hrabé de Angelis M. Rubio-Aliaga I, et al. PLoS One. 2009 Jun 29;4(6):e6054. doi: 10.1371/journal.pone.0006054. PLoS One. 2009. PMID: 19562077 Free PMC article. - Golgi-localized Ring Finger Protein 121 is necessary for MYCN-driven neuroblastoma tumorigenesis.
Cheung BB, Mittra R, Murray J, Wang Q, Seneviratne JA, Raipuria M, Wong IPL, Restuccia D, Gifford A, Salib A, Sutton S, Huang L, Ferdowsi PV, Tsang J, Sekyere E, Mayoh C, Luo L, Brown DL, Stow JL, Zhu S, Young RJ, Solomon BJ, Chappaz S, Kile B, Kueh A, Herold MJ, Hilton DJ, Liu T, Norris MD, Haber M, Carter DR, Parker MW, Marshall GM. Cheung BB, et al. Commun Biol. 2024 Oct 14;7(1):1322. doi: 10.1038/s42003-024-06899-8. Commun Biol. 2024. PMID: 39402275 Free PMC article. - Whole exome sequencing of ENU-induced thrombosis modifier mutations in the mouse.
Tomberg K, Westrick RJ, Kotnik EN, Cleuren AC, Siemieniak DR, Zhu G, Saunders TL, Ginsburg D. Tomberg K, et al. PLoS Genet. 2018 Sep 6;14(9):e1007658. doi: 10.1371/journal.pgen.1007658. eCollection 2018 Sep. PLoS Genet. 2018. PMID: 30188893 Free PMC article. - c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression.
Nakata Y, Shetzline S, Sakashita C, Kalota A, Rallapalli R, Rudnick SI, Zhang Y, Emerson SG, Gewirtz AM. Nakata Y, et al. Mol Cell Biol. 2007 Mar;27(6):2048-58. doi: 10.1128/MCB.01100-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242210 Free PMC article.
References
- Hrabe de Angelis, M. H., Flaswinkel, H., Fuchs, H., Rathkolb, B., Soewarto, D., Marschall, S., Heffner, S., Pargent, W., Wuensch, K., Jung, M., et al. (2000) Nat. Genet. 25, 444-447. - PubMed
- Kile, B. T., Hentges, K. E., Clark, A. T., Nakamura, H., Salinger, A. P., Liu, B., Box, N., Stockton, D. W., Johnson, R. L., Behringer, R. R., et al. (2003) Nature 425, 81-86. - PubMed
- Herron, B. J., Lu, W., Rao, C., Liu, S., Peters, H., Bronson, R. T., Justice, M. J., McDonald, J. D. & Beier, D. R. (2002) Nat. Genet. 30, 185-189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases