pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos - PubMed (original) (raw)
pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos
Angelike Stathopoulos et al. Genes Dev. 2004.
Abstract
The Heartless (Htl) FGF receptor is required for the differentiation of a variety of mesodermal tissues in the Drosophila embryo, yet its ligand is not known. Here we identify two new FGF genes, thisbe (ths) and pyramus (pyr), which probably encode the elusive ligands for this receptor. The two genes exhibit dynamic patterns of expression in epithelial tissues adjacent to Htl-expressing mesoderm derivatives, including the neurogenic ectoderm, stomadeum, and hindgut. Embryos that lack ths+ and pyr+ exhibit defects related to those seen in htl mutants, including delayed mesodermal migration during gastrulation and a loss of cardiac tissues and hindgut musculature. The misexpression of Ths in wild-type and mutant embryos suggests that FGF signaling is required for both cell migration and the transcriptional induction of cardiac gene expression. The characterization of htl and ths regulatory DNAs indicates that high levels of the maternal Dorsal gradient directly activate htl expression, whereas low levels activate ths. It is therefore possible to describe FGF signaling and other aspects of gastrulation as a direct manifestation of discrete threshold readouts of the Dorsal gradient.
Figures
Figure 1.
Thisbe and Pyramus are FGF-ligands. (A) RACE was done to isolate the 5′ sequence of the CG12443/Neu4 open reading frame (black) predicted by BDGP. Three previously unidentified exons were found that now place the Neu4 enhancer (open box) in the second intron of the thisbe gene (red). (B) An alignment of the putative protein sequences of D. melanogaster Thisbe and Pyramus with D. melanogaster Branchless, Human FGF8, and the PFAM Hidden Markov Model (HMM) consensus for the FGF protein family (top line). The HMM consensus represents the residues having the highest probability of occurrence for each position in an alignment of all known FGF proteins (Bateman et al. 2002). Dark highlighting indicates positions where 80% of proteins are identical; light highlighting indicates positions where 80% of the residues are similar using a PAM 250 substitution matrix. Intron positions are indicated with arrows. Black bars beneath the alignment indicate the positions of the 12 β strands in the FGF trefoil structure. (C) Reconstruction of phylogenetic relationships between the Drosophila FGF proteins Thisbe and Pyramus with other FGF proteins from fly, worm, fish, mosquito, virus, and human. Branch lengths are proportional to sequence divergence. The numbers at nodes are bootstrap values and indicate degree of support for particular branching relationships on a 1:100 scale. (A.gam) Anopheles gambiae; (D.mel) Drosophila melanogaster; (D.pse) Drosophila pseudoobscura; (Zebrafish) Danio rerio; (Human) Homo sapiens; (NPHV) Bombyx mori nuclear polyhedrosis virus. See Materials and Methods for accession numbers. (D) Genomic organization of pyramus and thisbe. pyr and ths (formerly CG13194 and CG12443, respectively) are linked genes separated by ∼80 kb. CG13195 and CG12444 (green boxes) encode related proteins that contain periplasmic binding domains. The light-blue shading indicates the proposed limits of a gene duplication event that resulted in these duplicated pairs of genes. Df(2R)BSC25 is a deficiency that has ∼200 kb removed, including the ∼110-kb interval containing the pyr and ths genes. The deletion also removes a number of flanking genes, including the Damm caspase. To date, none of these have been implicated in embryonic development. The horizontal lines at the bottom of the diagram indicate the flanking regions that are retained in the deficiency chromosome.
Figure 2.
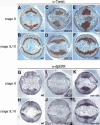
thisbe and pyramus expression patterns throughout embryogenesis. In situ hybridizations depicting ths (_A_-_D,I_-L) and pyr (_E_-_H,M_-P) localization in the embryo. For all embryos, anterior is to the left. Ventral views are depicted in C, I, and M. Lateral views are depicted for all other embryos with the dorsal surface on top. (A,E) Views of cellularized embryos (stage 5). Both pyr (E) and ths (A) are expressed in broad lateral stripes. (B,F) Ventral views of gastrulating embryos beginning rapid germ-band elongation (stage 7). pyr expression diminishes (F) while ths remains robust (B). (C) View of an embryo undergoing germ-band elongation (stage 8). ths expression remains strong throughout the neurogenic ectoderm but is excluded from the midline. (I) At later stages, ths expression is restricted to a subset of neuroblasts. In contrast, pyr expression is present only in select regions of the neurogenic ectoderm, specifically the dorsal region (G) and in distinct domains of the ventral region (M). (D,H,I,M) Lateral and ventral views of embryos that have completed germ-band elongation (stage 10). ths expression becomes limited to the dorsal neurogenic ectoderm (D) and then falls away almost completely from these dorsal ectodermal regions as well, with staining remaining within visceral mesoderm founder cells (J). (H) pyr is expressed in stripes throughout the embryo. (N) Later, pyr is expressed in 11 groups of cells within the ectoderm in proximity to cells of the mesoderm that will take on a pericardial fate. ths is also expressed in stripes and spots similar to pyr, but ths expression in these domains is only apparent after significant overstaining (data not shown). (K,O) Views of embryos undergoing germ-band retraction (stages 13-14). pyr and ths are expressed in regions flanking the proctodeum and stomodeum, the ectodermal derivatives of the gut, as well as the central nervous system. (L,P) Views of late-stage embryos having completed germ-band retraction (stages 15-17). ths and pyr expression remains associated with the foregut and the hindgut. Additional expression of ths can be observed in what appear to be the muscle attachment sites (L; arrowhead).
Figure 3.
Df(2R)BSC25 homozygotes exhibit delayed mesoderm spreading that is similar to Htl mutants. Embryos were stained with anti-Twist (_A_-F) or anti-dpERK antibodies (_G_-L) and sectioned as described (see Materials and Methods). (A,B,G,H) Wild-type embryos. (C,D,I,J) DfBSC25 mutant embryos. (E,F,) htl mutant embryos. (K,L) Embryos misexpressing ths in the mesoderm. Sections were made of embryos either in the early stage of mesoderm spreading, stage 8 (A,C,E,G,I,K), or once spreading in the wild type is complete, stage 9,10 (B,D,F, H,J,L). (_A_-F) Wild-type embryos with Twist staining showing early mesodermal cells beginning to migrate (A) and once migration is complete at later stages (B). In BSC25 deficiency and htl mutant embryos, mesoderm cells remain clumped (C,E) at a stage when wild-type cells have initiated migration (A), but do spread eventually and contact Dpp-expressing ectodermal cells (D,F) almost as fully as wild-type cells do (B). (_G_-L) dpERK staining in wild-type embryos is present in mesodermal cells in contact with the ectoderm early (G) and is limited later to only the cells at the leading edge of the migrating mesoderm (H). dpERK staining is absent from the mesodermal cells of BSC25 deficiency mutant embryos both early (I) and late (J). When ths is ectopically expressed in the mesoderm using a twi-Gal4 driver (twi > ths), dpERK staining is expanded to all cells of the entire mesoderm at both early (K) and late (L) stages.
Figure 4.
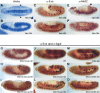
Df(2R)BSC25 homozygotes exhibit abnormalities similar to those seen in htl mutants. Embryos have undergone germ-band elongation. They are oriented with anterior to the left and dorsal up. (A,D,G,J) Wild-type embryos were stained with the anti-Eve antibody (A,D), tin and bap RNA probes (G,J, respectively). (A) Eve is expressed in approximately three pericardial cells within each hemisegment of the dorsal mesoderm. (D) Eve is also expressed in specific subsets of neurons within each neuromere of the developing ventral nerve cord. (G) tin mRNAs are expressed in two lineages of the dorsal mesoderm, the visceral mesoderm (arrow), and the cardiac mesoderm (arrowhead). (J) At this same stage, bap mRNA is expressed in a broad domain of the dorsal mesoderm. (B,E,H,K) Mutant embryos homozygous for a null mutation in htl. Eve staining is lost in the pericardial cells (B), but unaffected in the CNS (E). There are reduced levels of tin mRNAs (H) in the cardiac mesoderm (arrowhead), whereas staining in the visceral mesoderm is nearly normal (arrow). bap expression within the visceral mesoderm is also reduced in a subset of segments (K). (C,F,I,L) Mutant embryos homozygous for the BSC25 deficiency that removes the ths and pyr genes. These embryos exhibit phenotypes that are similar to those observed for htl mutants (B,E,H,K).
Figure 5.
FGF signaling is essential for the differentiation of specific muscle types in advanced-stage embryos. Embryos have undergone germ-band retraction and were stained with either a Mef2 antibody (_A_-I) or bap mRNA probe (_J_-L). (A,D,G,J) Wild-type embryos. (A) Mef2 is expressed in a variety of differentiating mesoderm lineages in retracted embryos, including the dorsal vessel (heart) and somatic body wall muscles. Mef2 is also expressed in the differentiating pharyngeal muscles associated with the stomadeum (asterisk in D), the hindgut musculature (arrowhead in D), and ventral oblique body wall muscles (white brackets in G). (J) bap mRNAs are detected in the pharyngeal muscles and hindgut musculature. (B,E,H,K) htl mutant embryos. (B) Mef2 staining indicates a loss of dorsal mesoderm derivatives, including the heart and dorsal mesoderm derivatives. (E) Mef2 staining is reduced in the pharynx (asterisk) and absent in the hindgut (arrowhead). (H) There is also a severe reduction in the number of ventral oblique muscles (brackets). (K) bap mRNAs are not expressed in the pharynx or hindgut musculature (cf. J). (C,F,I,L) BSC25 deficiency homozygotes. These embryos exhibit phenotypes that are similar to those observed for htl mutants (B,E,H,K).
Figure 6.
Misexpression of Ths causes a variety of mesoderm patterning defects. Embryos were stained using a ths ribo-probe (A,B), an anti-Eve antibody (_C,D,G_-O), or the anti-Mef2 antibody (E,F). (A,B) ths misexpression in the mesoderm using a twi-Gal4 driver and UAS-ths transgene. ths expression was detected by in situ hybridization and is misexpressed in the ventral mesoderm in cellularizing embryos (data not shown) and during the formation of the ventral furrow (A). Weaker staining can be seen in the neurogenic ectoderm (arrowheads in A), which corresponds to the expression of the endogenous gene. ths mRNAs persist in the mesoderm during elongation and shortening (B; data not shown). (C,D) Eve staining in embryos that overexpress ths using the twi-Gal4 driver. An expansion in the number of Eve-expressing pericardial cells is already detected at the completion of germ-band elongation, when the cardiac mesoderm is first induced (data not shown). Expanded Eve staining persists during germ-band shortening (C) and after the completion of germ-band retraction (D). (E,F) Mef2 staining in embryos that ectopically express ths using the twi-Gal4 driver. Staining is essentially normal during elongation (data not shown), but patterning defects can be seen at the completion of germ-band retraction (E,F). There is an expansion in the number of Mef2-expressing cells in dorsal mesoderm derivatives (E), and a block in the formation of ventral oblique muscles (brackets, F). Despite these abnormalities, most mesoderm lineages are formed, including the pharyngeal muscles (asterisk in E) and hindgut musculature (arrowhead in F). (_G_-O) Embryos were stained using antibodies against Eve and β-gal to distinguish mutant embryos from wild-type embryos, those carrying the ftz-lacZ balancer chromosome. Anti-β-gal staining in broad stripes corresponding to where ftz drives expression of lacZ can be observed in J and M, but this staining is absent from mutant embryos that do not contain the balancer chromosome (_G_-I,K,L,N,O). (_G_-I) Different forms of the Htl receptor were expressed in the mesoderm using twist-Gal4. The embryo in G is homozygous for the BSC25 deficiency and expresses a wild-type Htl transgene (UAS-Htl) in the mesoderm (twi > Htl). It lacks expression in the pericardial cells, but exhibits normal staining in the developing CNS. The embryos in H and I are also homozygous for the deficiency, but express the activated Htl receptor (UAS-actHtl) in the mesoderm (twi > actHtl). Eve staining is partially restored in the pericardial cells during germ-band shortening (arrows in H) and after retraction is complete (I). (J) Expression of Eve in a wild-type background is limited to about three cells per hemisegment. Broad stripes of expression can be seen that correspond to expression of β-gal by the ftz-lacZ transgene from the balancer chromosome. (K,L). Thisbe was expressed in the mesoderm of embryos homozygous for the BSC25 deficiency using the twist-Gal4 driver (BSC25; twi > ths). Eve expression is partially restored in the pericardial cells during germ-band shortening (K) and after retraction is complete (L). (_M_-O) Thisbe was expressed in the ectoderm of embryos using the 69B-Gal4 driver (69B > ths). (M) In wild-type embryos, a substantial expansion of pericardial cell specification is observed. When Thisbe is ectopically expressed in the ectoderm of embryos homozygous for the BSC25 deficiency using 69B-Gal4 (BSC25; 69B-Gal4), Eve expression is restored in the pericardial cells in a more uniform domain along the anterior-posterior axis during both germ-band shortening (N) and after retraction is complete (O).
Figure 7.
FGF signaling activation is a direct readout of Dorsal threshold outputs. A mesoderm enhancer was identified within the htl gene (A). The previously identified ths enhancer is shown in E. The htl enhancer is 800 bp, whereas the ths enhancer is 500 bp. Putative Dorsal-binding sites are indicated in the diagrams (dl), as well as additional sequence motifs, including Twist E-box recognition sequences (T) in the htl enhancer (A). The ths enhancer contains at least one high-affinity Snail repressor site that keeps the enhancer silent in the ventral mesoderm. (_B_-D) Transgenic embryos that contain the htl enhancer attached to a lacZ reporter gene. LacZ expression was detected by in situ hybridization. Staining is first detected in the ventral mesoderm during cellularization (B) and persists in the mesoderm during formation of the ventral furrow (C) and germ-band elongation (D). (_F_-H) Transgenic embryos that express the ths enhancer attached to a lacZ reporter gene. Staining is detected in cellularizing embryos (F) and persists in the ectoderm during the early stages of germ-band elongation (G). After elongation expression is diminished (H). (I) Diagrams showing different patterns of expression generated by the Dorsal gradient. The circles represent cross-sections through early embryos. The Dorsal nuclear gradient present at stage 5 is depicted in the diagram on the top. High levels of Dorsal activate Htl and Dof/Hbr/Sms, whereas low levels activate Ths and Pyr, as well as Sog. These same low levels of nuclear Dorsal also repress Dpp and restrict its expression to the dorsal ectoderm. After invagination of the mesoderm (stage 7,8), Ths and Pyr activate Htl signaling and thereby control the spreading of the mesoderm toward the dorsal ectoderm (middle diagram). Mesoderm that reaches the dorsal ectoderm is induced by Dpp to form cardiac tissues (bottom diagram).
Similar articles
- FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation.
Kadam S, McMahon A, Tzou P, Stathopoulos A. Kadam S, et al. Development. 2009 Mar;136(5):739-47. doi: 10.1242/dev.027904. Epub 2009 Jan 21. Development. 2009. PMID: 19158183 Free PMC article. - FGF signaling supports Drosophila fertility by regulating development of ovarian muscle tissues.
Irizarry J, Stathopoulos A. Irizarry J, et al. Dev Biol. 2015 Aug 1;404(1):1-13. doi: 10.1016/j.ydbio.2015.04.023. Epub 2015 May 6. Dev Biol. 2015. PMID: 25958090 Free PMC article. - Drosophila gastrulation: identification of a missing link.
Leptin M, Affolter M. Leptin M, et al. Curr Biol. 2004 Jun 22;14(12):R480-2. doi: 10.1016/j.cub.2004.06.016. Curr Biol. 2004. PMID: 15203022 Review. - The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator?
Bae YK, Trisnadi N, Kadam S, Stathopoulos A. Bae YK, et al. Cell Adh Migr. 2012 Sep-Oct;6(5):397-403. doi: 10.4161/cam.21103. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076054 Free PMC article. Review.
Cited by
- Lipid modification of secreted signaling proteins.
Miura GI, Treisman JE. Miura GI, et al. Cell Cycle. 2006 Jun;5(11):1184-8. doi: 10.4161/cc.5.11.2804. Epub 2006 Jun 1. Cell Cycle. 2006. PMID: 16721064 Free PMC article. Review. - Drosophila glypican Dally-like acts in FGF-receiving cells to modulate FGF signaling during tracheal morphogenesis.
Yan D, Lin X. Yan D, et al. Dev Biol. 2007 Dec 1;312(1):203-16. doi: 10.1016/j.ydbio.2007.09.015. Epub 2007 Sep 20. Dev Biol. 2007. PMID: 17959166 Free PMC article. - Cytonemes coordinate asymmetric signaling and organization in the Drosophila muscle progenitor niche.
Patel A, Wu Y, Han X, Su Y, Maugel T, Shroff H, Roy S. Patel A, et al. Nat Commun. 2022 Mar 4;13(1):1185. doi: 10.1038/s41467-022-28587-z. Nat Commun. 2022. PMID: 35246530 Free PMC article. - Myotube migration to cover and shape the testis of Drosophila depends on Heartless, Cadherin/Catenin, and myosin II.
Rothenbusch-Fender S, Fritzen K, Bischoff MC, Buttgereit D, Oenel SF, Renkawitz-Pohl R. Rothenbusch-Fender S, et al. Biol Open. 2017 Dec 15;6(12):1876-1888. doi: 10.1242/bio.025940. Biol Open. 2017. PMID: 29122742 Free PMC article. - Single-cell transcriptomics of the Drosophila wing disc reveals instructive epithelium-to-myoblast interactions.
Everetts NJ, Worley MI, Yasutomi R, Yosef N, Hariharan IK. Everetts NJ, et al. Elife. 2021 Mar 22;10:e61276. doi: 10.7554/eLife.61276. Elife. 2021. PMID: 33749594 Free PMC article.
References
- Ahmad S.M. and Baker, B.S. 2002. Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109: 651-661. - PubMed
- Anderson K.V., Jurgens, G., and Nusslein-Volhard, C. 1985. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell 42: 779-789. - PubMed
- Azpiazu N. and Frasch, M. 1993. tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes & Dev. 7: 1325-1340. - PubMed
- Beiman M., Shilo, B.Z., and Volk, T. 1996. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes & Dev. 10: 2993-3002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM46638/GM/NIGMS NIH HHS/United States
- GM20352/GM/NIGMS NIH HHS/United States
- F32 GM020352/GM/NIGMS NIH HHS/United States
- R01 HD030832/HD/NICHD NIH HHS/United States
- HD30832/HD/NICHD NIH HHS/United States
- R01 GM046638/GM/NIGMS NIH HHS/United States
- R37 GM046638/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases