Role of cytoplasmic domain serines in intracellular trafficking of furin - PubMed (original) (raw)
Role of cytoplasmic domain serines in intracellular trafficking of furin
Florencia B Schapiro et al. Mol Biol Cell. 2004 Jun.
Abstract
Furin is a transmembrane protein that cycles between the plasma membrane, endosomes, and the trans-Golgi network, maintaining a predominant distribution in the latter. It has been shown previously that Tac-furin, a chimeric protein expressing the extracellular and transmembrane domains of the interleukin-2 receptor alpha chain (Tac) and the cytoplasmic domain of furin, is delivered from the plasma membrane to the TGN through late endosomes, bypassing the endocytic recycling compartment. Tac-furin also recycles in a loop between the TGN and late endosomes. Localization of furin to the TGN is modulated by a six-amino acid acidic cluster that contains two phosphorylatable serines (SDSEED). We investigated the role of these serines in the trafficking of Tac-furin by using a mutant chimera in which the SDS sequence was replaced by the nonphosphorylatable sequence ADA (Tac-furin/ADA). Although the mutant construct is internalized and delivered to the TGN, both the postendocytic trafficking and the steady-state distribution were found to differ from the wild-type. In contrast with Tac-furin, Tac-furin/ADA does not enter late endosomes after being internalized. Instead, it traffics with transferrin to the endocytic recycling compartment, and from there it is delivered to the TGN. As with Tac-furin, Tac-furin/ADA is sorted from the TGN into late endosomes at steady state, but its retrieval from the late endosomes to the TGN is inhibited. These results suggest that serine phosphorylation plays an important role in at least two steps of Tac-furin trafficking, acting as an active sorting signal that mediates the selective sorting of Tac-furin into late endosomes after internalization, as well as its retrieval from late endosomes back to the TGN.
Figures
Figure 1.
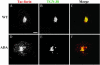
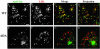
Postendocytic and steady-state distribution of Tac-furin and Tac-furin/ADA in TRVb-1 cells. Cells expressing either the Tac-furin or the Tac-furin/ADA construct (ADA) were incubated for 5 min with Ax546–anti-Tac antibodies, washed and chased for 5 min (A–D) or 2 h (E–H) before fixation. Confocal projections obtained at a high (A, B, E and F) and low (C, D, G, and H) magnification are shown. Bar (A, B, E, and F), 10 μm. Bar (C, D, G, and H), 5 μm.
Figure 2.
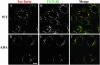
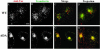
Tac-furin/ADA localizes to the TGN at steady state. Cells were incubated for 5 min with Ax546–anti-Tac (A and D), washed, and chased for 2 h. Cells were then fixed, permeabilized, and labeled with polyclonal antibodies against TGN-38, followed by Ax488–anti-rabbit antibodies (B and E). (C and F) Merge of the anti-Tac signal (red) and the TGN38 signal (green). Colocalized areas range from orange to yellow. Single confocal planes are shown. Bar, 10 μm.
Figure 3.
Tac-furin/ADA localizes to TGN fragments after nocodazole treatment. Cells were labeled for 15 min with Ax546–anti-Tac (A and D), washed, and chased for 2 h. Labeled cells were incubated with nocodazole (33 μM) at 4°C for 30 min and then for another 30 min at 37°C, fixed, permeabilized and stained with anti-TGN38, as described in Figure 2 (B and E). C and F show the merge of the anti-Tac signal (red) and the TGN38 signal (green). Colocalized areas range from orange to yellow. Single confocal planes are shown. Bar, 10 μm.
Figure 4.
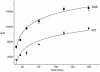
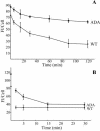
Accumulation of 125I-anti-Tac antibody in WT and ADA mutant cell lines. Cells were incubated with 125I-anti-Tac antibody for 5, 15, 30, 60, 120, or 180 min at 37°C, washed extensively with ice-cold medium 1 with 1% (wt/vol) BSA, and solubilized. Cell-associated radioactivity corresponding to each time point was measured. Error bars represent SEM.
Figure 5.
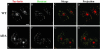
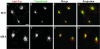
Tac-furin/ADA is sorted into late endosomes at steady state. Late endosomes were labeled by preincubating cells with FITC-dextran (B and F) for 6 h. Cells were then washed, pulsed with Ax546–anti-Tac (A and E) for 5 min, and chased for 2 h before fixation. FITC-dextran was present in the medium throughout the pulse and the first hour of the chase. (C and G) Merge of the anti-Tac signal (red) and the dextran signal (green). A–C and E–G show single confocal planes. A projection of successive confocal planes is shown in D and H. Colocalized areas range from orange to yellow. Bar, 10 μm.
Figure 6.
Retrieval of Tac-furin/ADA from late endosomes back to the TGN at steady state is significantly inhibited. Cells were incubated for 5 min with Ax488–anti-Tac, washed, and chased for 2 h. Selective bleaching of the Golgi complex of labeled cells was then performed on a LSM 510 by scanning with high-intensity illumination (100% transmittance). The recovery of fluorescence in the bleached region was monitored at 2, 5, 15, 30, and 45 min thereafter. A–F. Single confocal planes showing the fluorescence distribution immediately before bleaching (prebleaching, A and D), immediately after bleaching (postbleaching, B and E) and 45 min after bleaching (recovery, C and F). Bar, 10 μm. (G) Kinetics of Tac-furin/ADA retrieval from late endosomes back to the TGN at steady state. For the imaged focal plane, total cell-associated fluorescence (FT) and fluorescence of the bleached area (FG) at the different time points were quantified and plotted as FT/FG versus time. Also represented are the levels of Tac-furin and Tac-furin/ADA fluorescence that localized to the Golgi within the selected focal plane before photobleaching. Data points represent the means of two independent experiments. Error bars represent SEM.
Figure 7.
Internalized Tac-furin/ADA is delivered to the TGN without accumulation into late endosomes. Cells were incubated with Ax488-anti-Tac (A and E) and DiI-LDL (B and F) for 5 min, washed, chased for 10 min, and fixed. After 10-min chase, most of the LDL should be in late endosomes. (C and G) Merge of the anti-Tac signal (green) and the LDL signal (red). A–C and E–G show single confocal planes. A projection of successive confocal planes is shown in D and H. Colocalized areas range from orange to yellow. Bar, 10 μm.
Figure 8.
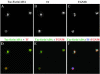
Internalized Tac-furin/ADA enters the ERC en route to the TGN. Cells were incubated with Ax546-anti-Tac (A and E) and with Ax488-transferrin (B and F) for 5 min, washed and chased for 1 min in the presence of Ax488–transferrin. Panels C and G show the merge of the anti-Tac signal (red) and the transferrin signal (green). Panels A–C and E–G show single confocal planes. A projection of successive confocal planes is shown in D and H. Colocalized areas range from orange to yellow. Bar, 10 μm.
Figure 9.
Internalized Tac-furin/ADA starts to segregate from the ERC into the TGN as early as 15 min after being internalized. Cells were incubated with Ax546-anti-Tac (A and E) and Ax488–transferrin (B and F) for 5 min, washed, and chased for 10 min in the presence of Ax488-transferrin. C and G show the merge of the anti-Tac signal (red) and the transferrin signal (green). A–C and E–G show single confocal planes. A projection of successive confocal planes is shown in D and H. Colocalized areas range from orange to yellow. Bar, 10 μm.
Figure 10.
Most of Tac-furin/ADA localizes to the ERC after 10 min of being internalized. Cells were incubated with Ax488-anti-Tac (A) and Ax633-transferrin (Tf, B) for 5 min, washed and chased for 10 min in the presence of Ax633–transferrin. Cells were then fixed, permeabilized, and labeled with polyclonal antibodies against TGN-38, followed by Cy3-anti rabbit antibodies (C). (D) Merge of the anti-Tac signal (green) and the transferrin signal (red). (E) Merge of the anti-Tac signal (green) and the TGN38 signal (red). (F) Merge of the anti-Tac signal (green), the transferrin signal (blue) and the TGN38 signal (red). Single confocal planes are shown. Bar, 10 μm.
Figure 11.
Postendocytic and steady-state retrieval of Tac-furin/ADA back to the plasma membrane after internalization. (A) A portion of Tac-furin/ADA is retrieved back to the plasma membrane after internalization. Cells were incubated with Ax488–anti-Tac for 5 min, washed and chased for 2, 5, 15, and 30 min in the presence of the quenching antibody anti-Ax488. (B) Sorting of Tac-furin/ADA from the TGN back to the plasma membrane at steady state is inhibited. Cells were incubated with Ax488–anti-Tac for 15 min, washed and chased for 2 h. Labeled cells were further incubated in the presence of anti-Ax488 for 5, 15, 30, 60, 90, and 120 min. In both sets of experiments, cell-associated fluorescence was quantified as described in MATERIALS AND METHODS. Data points represent the means of two independent experiments. Error bars indicate SEM.
Figure 12.
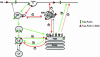
Model for the transport of Tacfurin and the mutant construct Tac-furin/ADA. Both constructs are internalized from the plasma membrane and initially enter sorting endosomes (SE). Tac-furin is then retained in the sorting endosomes as they mature into late endosomes (LE). Shortly after the endosomes lose the characteristics of sorting endosomes, Tac-furin is delivered from the maturing endosomes into the TGN. This may occur in the maturing endosome/endosome carrier vesicle (Gruenberg and Maxfield, 1995). In contrast, Tac-furin/ADA is removed rapidly from sorting endosomes and transported to the ERC. Although a portion of the mutant construct is recycled back to the cell surface, the rest is delivered to the TGN as is the case for TGN38. Black arrows represent steps of protein traffic that exist, but are not followed by Tac-furin nor Tac-furin/ADA. The stippled TGN-PM arrow represents decreased retrieval of Tac-furin/ADA from the TGN to the PM.
Similar articles
- Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways.
Mallet WG, Maxfield FR. Mallet WG, et al. J Cell Biol. 1999 Jul 26;146(2):345-59. doi: 10.1083/jcb.146.2.345. J Cell Biol. 1999. PMID: 10465644 Free PMC article. - Rab9-dependent retrograde transport and endosomal sorting of the endopeptidase furin.
Chia PZ, Gasnereau I, Lieu ZZ, Gleeson PA. Chia PZ, et al. J Cell Sci. 2011 Jul 15;124(Pt 14):2401-13. doi: 10.1242/jcs.083782. Epub 2011 Jun 21. J Cell Sci. 2011. PMID: 21693586 Free PMC article. - Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin.
Ikonomov OC, Fligger J, Sbrissa D, Dondapati R, Mlak K, Deeb R, Shisheva A. Ikonomov OC, et al. J Biol Chem. 2009 Feb 6;284(6):3750-61. doi: 10.1074/jbc.M806539200. Epub 2008 Dec 4. J Biol Chem. 2009. PMID: 19056739 Free PMC article. - Plant endosomes as protein sorting hubs.
González Solís A, Berryman E, Otegui MS. González Solís A, et al. FEBS Lett. 2022 Sep;596(17):2288-2304. doi: 10.1002/1873-3468.14425. Epub 2022 Jun 17. FEBS Lett. 2022. PMID: 35689494 Review. - From endosomes to the trans-Golgi network.
Lu L, Hong W. Lu L, et al. Semin Cell Dev Biol. 2014 Jul;31:30-9. doi: 10.1016/j.semcdb.2014.04.024. Epub 2014 Apr 23. Semin Cell Dev Biol. 2014. PMID: 24769370 Review.
Cited by
- Sorting nexin 17 facilitates LRP recycling in the early endosome.
van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G. van Kerkhof P, et al. EMBO J. 2005 Aug 17;24(16):2851-61. doi: 10.1038/sj.emboj.7600756. Epub 2005 Jul 28. EMBO J. 2005. PMID: 16052210 Free PMC article. - Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes.
Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Ishizaki R, et al. Mol Biol Cell. 2008 Jun;19(6):2650-60. doi: 10.1091/mbc.e07-10-1067. Epub 2008 Apr 16. Mol Biol Cell. 2008. PMID: 18417613 Free PMC article. - Full-length, membrane-anchored TWEAK can function as a juxtacrine signaling molecule and activate the NF-kappaB pathway.
Brown SA, Ghosh A, Winkles JA. Brown SA, et al. J Biol Chem. 2010 Jun 4;285(23):17432-41. doi: 10.1074/jbc.M110.131979. Epub 2010 Apr 12. J Biol Chem. 2010. PMID: 20385556 Free PMC article. - Signaling from the secretory granule to the nucleus.
Rajagopal C, Mains RE, Eipper BA. Rajagopal C, et al. Crit Rev Biochem Mol Biol. 2012 Jul-Aug;47(4):391-406. doi: 10.3109/10409238.2012.694845. Epub 2012 Jun 8. Crit Rev Biochem Mol Biol. 2012. PMID: 22681236 Free PMC article. Review. - ARF1 and ARF4 regulate recycling endosomal morphology and retrograde transport from endosomes to the Golgi apparatus.
Nakai W, Kondo Y, Saitoh A, Naito T, Nakayama K, Shin HW. Nakai W, et al. Mol Biol Cell. 2013 Aug;24(16):2570-81. doi: 10.1091/mbc.E13-04-0197. Epub 2013 Jun 19. Mol Biol Cell. 2013. PMID: 23783033 Free PMC article.
References
- Díaz, E., and Pfeffer, S.R. (1998). TIP 47, a cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93, 433-443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous