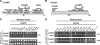Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor - PubMed (original) (raw)
Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor
Cecilia N Arighi et al. J Cell Biol. 2004 Apr.
Abstract
The cation-independent mannose 6-phosphate receptor (CI-MPR) mediates sorting of lysosomal hydrolase precursors from the TGN to endosomes. After releasing the hydrolase precursors into the endosomal lumen, the unoccupied receptor returns to the TGN for further rounds of sorting. Here, we show that the mammalian retromer complex participates in this retrieval pathway. The hVps35 subunit of retromer interacts with the cytosolic domain of the CI-MPR. This interaction probably occurs in an endosomal compartment, where most of the retromer is localized. In particular, retromer is associated with tubular-vesicular profiles that emanate from early endosomes or from intermediates in the maturation from early to late endosomes. Depletion of retromer by RNA interference increases the lysosomal turnover of the CI-MPR, decreases cellular levels of lysosomal hydrolases, and causes swelling of lysosomes. These observations indicate that retromer prevents the delivery of the CI-MPR to lysosomes, probably by sequestration into endosome-derived tubules from where the receptor returns to the TGN.
Figures
Figure 1.
hVps35 binds to the cytosolic domain of the CI-MPR. (A) Schematic representation of the cytosolic domain of the CI-MPR indicating binding sites for interaction partners involved in sorting. (B) Schematic representation of hVps35 indicating segments that bind to other retromer subunits and to the CI-MPR. (C and D) Yeast two-hybrid analyses showing the interaction of full-length hVps35 with segments of the CI-MPR cytosolic domain, and the interaction of the full-length CI-MPR cytosolic domain with fragments of hVps35. Interaction of the fusion proteins was detected by growth of cotransformed cells in the absence of leucine (−Leu) as described in the Materials and methods. hVps29 and lamin C were used as negative controls.
Figure 2.
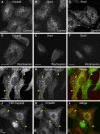
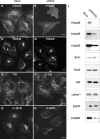
Immunofluorescence microscopy analysis of the localization of retromer subunits. (A–F) HeLa cells were incubated for 30 min at 37°C in the absence (A–C) or presence (D–F) of 100 nM wortmannin. Cells were then fixed and stained with rabbit pAbs to endogenous hVps26 (A and D), Snx1 (B and E), or Snx2 (C and F), followed by donkey Alexa® 488–conjugated anti–rabbit Igs. (G–I) HeLa cells transiently transfected with myc-Snx2 were costained for endogenous hVps26 as described above (G), and for myc-Snx2 using a mouse mAb to the myc epitope and donkey Cy3-conjugated anti–mouse Igs (H). (J–L) M1 cells stably transfected with YFP-hVps35 were stained for hVps26 as described above. Arrowheads indicate structures where hVps26 colocalizes with myc-Snx2 (G–I) and with YFP-hVps35 (J–L). Bars, 10 μm.
Figure 3.
Colocalization of hVps26 with organellar markers. HeLa cells were fixed and costained with a rabbit pAb to hVps26 (A, D, G, J, and M), and with mouse mAbs to the TfR (B), TGN46 (H), Lamp-1 (K), and the CI-MPR (N), followed by donkey Alexa® 488–conjugated anti–rabbit and donkey Cy3-conjugated anti–mouse Igs. CFP-Rab5Q79L was expressed by transient transfection (E). Arrowheads indicate structures where two proteins colocalize. Bars, 10 μm.
Figure 4.
Immuno-EM localization of hVps26 and TfR. Ultrathin cryosections of HeLa cells were immunogold labeled with anti-hVps26 (A) or double-immunogold labeled with anti-hVps26 (10-nm gold particles) and anti-TfR (B and C, 15-nm gold particles). Arrows indicate the localization of hVps26 to tubular structures; large arrowheads indicate the presence of a bilayered clathrin coat on multivesicular endosomes; small arrowheads show the presence of hVps26 at the neck of tubules originating from endosomes. TfR colocalizes with hVps26 both in the tubules and in the endosomes. Bars, 100 nm.
Figure 5.
Immuno-EM localization of hVps26 relative to CI-MPR and Lamp-1. Ultrathin cryosections of HeLa cells were double-immunogold labeled with anti-hVpS26 (10-nm gold particles) and either anti-CI-MPR or Lamp-1 (15-nm gold particles) (A and B, respectively). Notice the partial colocalization of hVps26 with CI-MPR in multivesicular endosomes (A). The endosomes that are labeled with hVps26 are devoid of Lamp-1 (B), pointing to the early endosomal nature of these structures. Bars, 100 nm.
Figure 6.
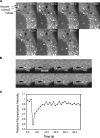
Fluorescence imaging of YFP-hVps35 in live cells. (A) Live M1 cells stably expressing YFP-hVps35 were imaged by confocal microscopy at 37°C. Notice the presence of YFP-hVps35 in punctate and tubular structures that are often associated with dark vacuolar profiles. The tubule marked by the arrowhead extends and detaches from a vacuole, and then heads toward the juxtanuclear area. The dark vacuole moves along with the YFP-hVps35–positive structure. The times at which each image was collected are indicated. (B) M1 cells stably expressing YFP-hVps35 were pretreated with 10 μM nocodazole for 1 h at 37°C before FRAP. 100% power in the 488-nm line was applied for photobleaching in the region indicated by the arrow. Recovery was monitored over time. The sequence shown represents frames taken every 2 s. Bar, 10 μm. (C) Quantification of the data in B shows recovery with a half-time of 6.5 s.
Figure 7.
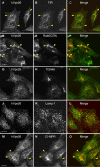
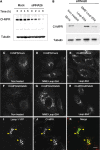
Effect of RNA-mediated interference of hVps26 on the expression of retromer subunits. HeLa cells were either mock treated or treated with hVps26 siRNA (siRNA) for 72 h, and were then analyzed by immunofluorescence microscopy (A–H) and immunoblotting (I). Immunofluorescence microscopy was performed with antibodies directed to endogenous hVps26 (A and B), TGN46 (C and D), TfR (E and F), or CI-MPR (G and H), all followed by the corresponding secondary antibodies labeled with either Alexa® 488 or Cy3. Bars, 10 μm. Immunoblots were probed with antibodies to the proteins indicated in I and are described in more detail in the Materials and methods.
Figure 8.
Lysosomal degradation of the CI-MPR upon depletion of retromer. (A) Immunoblot analysis of CI-MPR half-life in mock- and hVps26-siRNA–treated HeLa cells after treatment with 40 μg/ml cycloheximide. Tubulin was used as a control. (B) Immunoblot analysis of CI-MPR levels after silencing hVps26. HeLa cells were incubated in the absence or presence of the lysosomal inhibitors leupeptin (Leup, 1 mg/ml), E64 (5 μg/ml), and methionine methyl ester (MME, 10 mM) for 3 h, as indicated in the figure. Blots were probed with antibodies to the CI-MPR or tubulin (control). (C–K) Immunofluorescence microscopy of mock-treated (C–E) or hVps26-siRNA–treated HeLa cells (F–K) incubated in the absence or presence of the lysosomal inhibitors described in A, and stained with the antibody to the CI-MPR and donkey Cy3-conjugated anti–mouse Igs. Lamp-1-YFP (I–K) was expressed by transient transfection. In this case, hVps26-depleted cells were treated with MME/Leup/E64 protease inhibitors. Arrowheads in I–K indicate colocalization of Lamp-1-YFP with CI-MPR. Bar, 10 μm.
Figure 9.
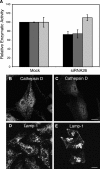
Lysosomal alterations in retromer-depleted cells. (A) Quantification of β-glucuronidase (black bars), β-hexosaminidase (dark gray bars), and aconitase (light gray bars) activities in lysates from mock-treated and hVps26-depleted HeLa cells. The results are expressed as the mean ± SD of at least three experiments, each of which was performed in triplicate. (B) Immunofluorescence microscopy of mock-treated (B and D) and hVps26-siRNA–treated (C and E) cells stained with an antibody to cathepsin D and donkey Alexa® 488–conjugated anti–rabbit Igs (B and C), or an antibody to Lamp-1 and donkey Cy3-conjugated anti–mouse Igs (D and E). Bars, 10 μm.
Similar articles
- Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer.
Seaman MN. Seaman MN. J Cell Biol. 2004 Apr;165(1):111-22. doi: 10.1083/jcb.200312034. J Cell Biol. 2004. PMID: 15078902 Free PMC article. - The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network.
Bujny MV, Popoff V, Johannes L, Cullen PJ. Bujny MV, et al. J Cell Sci. 2007 Jun 15;120(Pt 12):2010-21. doi: 10.1242/jcs.003111. J Cell Sci. 2007. PMID: 17550970 - A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer.
Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. Wassmer T, et al. J Cell Sci. 2007 Jan 1;120(Pt 1):45-54. doi: 10.1242/jcs.03302. Epub 2006 Dec 5. J Cell Sci. 2007. PMID: 17148574 - Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.
Kametaka S, Waguri S. Kametaka S, et al. Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review. - Recent advances in retromer biology.
McGough IJ, Cullen PJ. McGough IJ, et al. Traffic. 2011 Aug;12(8):963-71. doi: 10.1111/j.1600-0854.2011.01201.x. Epub 2011 May 23. Traffic. 2011. PMID: 21463457 Review.
Cited by
- Biogenesis of endosome-derived transport carriers.
Chi RJ, Harrison MS, Burd CG. Chi RJ, et al. Cell Mol Life Sci. 2015 Sep;72(18):3441-3455. doi: 10.1007/s00018-015-1935-x. Epub 2015 May 29. Cell Mol Life Sci. 2015. PMID: 26022064 Free PMC article. Review. - Sorting of the yeast vacuolar-type, proton-translocating ATPase enzyme complex (V-ATPase): identification of a necessary and sufficient Golgi/endosomal retention signal in Stv1p.
Finnigan GC, Cronan GE, Park HJ, Srinivasan S, Quiocho FA, Stevens TH. Finnigan GC, et al. J Biol Chem. 2012 Jun 1;287(23):19487-500. doi: 10.1074/jbc.M112.343814. Epub 2012 Apr 11. J Biol Chem. 2012. PMID: 22496448 Free PMC article. - Arf1-dependent LRBA recruitment to Rab4 endosomes is required for endolysosome homeostasis.
Szentgyörgyi V, Lueck LM, Overwijn D, Ritz D, Zoeller N, Schmidt A, Hondele M, Spang A, Bakhtiar S. Szentgyörgyi V, et al. J Cell Biol. 2024 Nov 4;223(11):e202401167. doi: 10.1083/jcb.202401167. Epub 2024 Sep 26. J Cell Biol. 2024. PMID: 39325073 Free PMC article. - RAB-6.2 and the retromer regulate glutamate receptor recycling through a retrograde pathway.
Zhang D, Isack NR, Glodowski DR, Liu J, Chen CC, Xu XZ, Grant BD, Rongo C. Zhang D, et al. J Cell Biol. 2012 Jan 9;196(1):85-101. doi: 10.1083/jcb.201104141. Epub 2012 Jan 2. J Cell Biol. 2012. PMID: 22213799 Free PMC article. - A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica.
Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. Nakada-Tsukui K, et al. Mol Biol Cell. 2005 Nov;16(11):5294-303. doi: 10.1091/mbc.e05-04-0283. Epub 2005 Aug 24. Mol Biol Cell. 2005. PMID: 16120649 Free PMC article.
References
- Chen, H.J., J. Remmler, J.C. Delaney, D.J. Messner, and P. Lobel. 1993. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor. A consensus casein kinase II site followed by 2 leucines near the carboxyl terminus is important for intracellular targeting of lysosomal enzymes. J. Biol. Chem. 268:22338–22346. - PubMed
- Chen, H.J., J. Yuan, and P. Lobel. 1997. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem. 272:7003–7012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous