The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway - PubMed (original) (raw)
The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway
Bernard Clantin et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Filamentous hemagglutinin (FHA), the major 230-kDa adhesin of the whooping cough agent Bordetella pertussis, is one of the most efficiently secreted proteins in Gram-negative bacteria. FHA is secreted by means of the two-partner secretion (TPS) pathway. Several important human, animal, and plant pathogens also secrete adhesins and other virulence factors by using this mode of secretion. A TPS system is composed of two separate proteins, with TpsA the secreted protein and TpsB its associated specific outermembrane transporter. All TPS-secreted proteins contain a distinctive N-proximal module essential for secretion, the TPS domain. We report here the 1.7- A structure of a functionally secreted 30-kDa N-terminal fragment of FHA. It reveals that the TPS domain folds into a beta-helix, with three extrahelical motifs, a beta-hairpin, a four-stranded beta-sheet, and an N-terminal capping, mostly formed by the nonconserved regions of the TPS domain. The structure thus explains why the TPS domain is able to initiate folding of the beta-helical motifs that form the central domain of the adhesin, because it is itself a beta-helical scaffold. It also contains less conserved extrahelical regions most likely involved in specific properties, such as the recognition of the outer-membrane transporter. This structure is representative of the TPS domains found so far in >100 secreted proteins from pathogenic bacteria. It also provides a mechanistic insight into how protein folding may be linked to secretion in the TPS pathway.
Figures
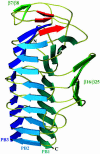
Fig. 1.
Ribbon representation of the overall structure of Fha30. The helix β-sheets PB1, PB2, and PB3, the β-hairpin β7/β8, and the β-sheet β16/β25 are shown. The three β-strands, β1, β2, and β3, involved in the N-terminal β-helix capping are represented in red. N- and C-terminal extremities are also indicated.
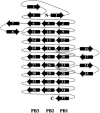
Fig. 2.
Diagram illustrating the topology of Fha30. PB1, PB2, PB3, and extra β-helical motifs are represented.
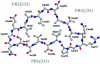
Fig. 3.
Ball-and-stick view of the coil formed by β-strands β31, β32, and β33 that belong to PB1, PB2, and PB3, respectively. This coil corresponds to the first R1 repeat and displays an isosceles-triangular-shaped section. The hydrogen bonds between the coil's main chain and the side chain of Ser-257 are illustrated.
Fig. 4.
Ribbon representation of the N-terminal capping that shields the β-helix hydrophobic interior from the solvent. The helix β-sheets PB1, PB2, and PB3, the β-hairpin β7/β8, and the β-sheet β16/β25 are shown. The three β-strands β1, β2, and β3 involved in the capping are represented in red.
Fig. 5.
Sequence alignment of representative TpsA proteins. Secondary structure elements observed in the Fha30 structure are indicated. The conserved residues are boxed in black (cut off at 60% conservation), and the homologous residues are boxed in gray. The conserved regions C1 and C2 are delimited.
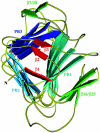
Fig. 6.
Ribbon view of Fha30 illustrating the LC1, C1, LC2, and C2 regions of the TPS domain. The R1 repeats that belong to the central right-handed β-helix domain of the full-length adhesin are also shown.
Similar articles
- Secretion signal of the filamentous haemagglutinin, a model two-partner secretion substrate.
Hodak H, Clantin B, Willery E, Villeret V, Locht C, Jacob-Dubuisson F. Hodak H, et al. Mol Microbiol. 2006 Jul;61(2):368-82. doi: 10.1111/j.1365-2958.2006.05242.x. Epub 2006 Jun 12. Mol Microbiol. 2006. PMID: 16771844 - The turn of the screw: variations of the abundant beta-solenoid motif in passenger domains of Type V secretory proteins.
Kajava AV, Steven AC. Kajava AV, et al. J Struct Biol. 2006 Aug;155(2):306-15. doi: 10.1016/j.jsb.2006.01.015. Epub 2006 May 4. J Struct Biol. 2006. PMID: 16765057 - Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily.
Clantin B, Delattre AS, Rucktooa P, Saint N, Méli AC, Locht C, Jacob-Dubuisson F, Villeret V. Clantin B, et al. Science. 2007 Aug 17;317(5840):957-61. doi: 10.1126/science.1143860. Science. 2007. PMID: 17702945 - Bordetella Filamentous Hemagglutinin, a Model for the Two-Partner Secretion Pathway.
Nash ZM, Cotter PA. Nash ZM, et al. Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.psib-0024-2018. doi: 10.1128/microbiolspec.PSIB-0024-2018. Microbiol Spectr. 2019. PMID: 30927348 Free PMC article. Review. - Protein secretion through autotransporter and two-partner pathways.
Jacob-Dubuisson F, Fernandez R, Coutte L. Jacob-Dubuisson F, et al. Biochim Biophys Acta. 2004 Nov 11;1694(1-3):235-57. doi: 10.1016/j.bbamcr.2004.03.008. Biochim Biophys Acta. 2004. PMID: 15546669 Review.
Cited by
- Structural determinants of the interaction between the TpsA and TpsB proteins in the Haemophilus influenzae HMW1 two-partner secretion system.
Grass S, Rempe KA, St Geme JW 3rd. Grass S, et al. J Bacteriol. 2015 May;197(10):1769-80. doi: 10.1128/JB.00039-15. Epub 2015 Mar 16. J Bacteriol. 2015. PMID: 25777673 Free PMC article. - Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils.
Mobberley-Schuman PS, Weiss AA. Mobberley-Schuman PS, et al. Infect Immun. 2005 Nov;73(11):7317-23. doi: 10.1128/IAI.73.11.7317-7323.2005. Infect Immun. 2005. PMID: 16239529 Free PMC article. - Marine prebiotics mediate decolonization of Pseudomonas aeruginosa from gut by inhibiting secreted virulence factor interactions with mucins and enriching Bacteroides population.
Janapatla RP, Dudek A, Chen CL, Chuang CH, Chien KY, Feng Y, Yeh YM, Wang YH, Chang HJ, Lee YC, Chiu CH. Janapatla RP, et al. J Biomed Sci. 2023 Feb 2;30(1):9. doi: 10.1186/s12929-023-00902-w. J Biomed Sci. 2023. PMID: 36732731 Free PMC article. - A novel secretion pathway of Salmonella enterica acts as an antivirulence modulator during salmonellosis.
Gal-Mor O, Gibson DL, Baluta D, Vallance BA, Finlay BB. Gal-Mor O, et al. PLoS Pathog. 2008 Apr 4;4(4):e1000036. doi: 10.1371/journal.ppat.1000036. PLoS Pathog. 2008. PMID: 18389060 Free PMC article. - A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence.
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J. Gottig N, et al. PLoS One. 2009;4(2):e4358. doi: 10.1371/journal.pone.0004358. Epub 2009 Feb 4. PLoS One. 2009. PMID: 19194503 Free PMC article.
References
- Henderson, I. R., Navarro-Garcia, F. & Nataro, J. P. (1998) Trends Microbiol. 6, 370-378. - PubMed
- Jacob-Dubuisson, F., Locht, C. & Antoine, R. (2001) Mol. Microbiol. 40, 306-313. - PubMed
- Yen, M.-R., Peabody, C. R., Partovi, S. M., Zhai, Y., Tseng, Y.-H. & Saier, M. H. (2002) Biochim. Biophys. Acta 1562, 6-31. - PubMed
- Schonherr, R., Tsolis, R., Focareta, T. & Braun, V. (1993) Mol. Microbiol. 9, 1229-1237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources