A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A - PubMed (original) (raw)
A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A
Hsin-Sheng Yang et al. Mol Cell Biol. 2004 May.
Abstract
An alpha-helical MA-3 domain appears in several translation initiation factors, including human eukaryotic translation initiation factor 4G (eIF4G) and DAP-5/NAT1/p97, as well as in the tumor suppressor Pdcd4. The function of the MA-3 domain is, however, unknown. C-terminal eIF4G (eIG4Gc) contains an MA-3 domain that is located within the eIF4A-binding region, suggesting a role for eIF4A binding. Interestingly, C-terminal DAP-5/NAT1/p97 contains an MA-3 domain, but it does not bind to eIF4A. Mutation of amino acid residues conserved between Pdcd4 and eIF4Gc but not in DAP-5/NAT1/p97 to the amino acid residues found in the DAP-5/NAT1/p97 indicates that some of these amino acid residues within the MA-3 domain are critical for eIF4A-binding activity. Six Pdcd4 mutants (Pdcd4(E249K), Pdcd4(D253A), Pdcd4(D414K), Pdcd4(D418A), Pdcd4(E249K,D414K), and Pdcd4(D253A,D418A)) lost >90% eIF4A-binding activity. Mutation of the corresponding amino acid residues in the eIF4Gc also produced similar results, as seen for Pdcd4. These results demonstrate that the MA-3 domain is important for eIF4A binding and explain the ability of Pdcd4 or eIF4Gc but not DAP-5/NAT1/p97 to bind to eIF4A. Competition experiments indicate that Pdcd4 prevents ca. 60 to 70% of eIF4A binding to eIF4Gc at a Pdcd4/eIF4A ratio of 1:1, but mutants Pdcd4(D253A) and Pdcd4(D253A,D418A) do not. Translation of stem-loop structured mRNA is susceptible to inhibition by wild-type Pdcd4 but not by Pdcd4(D253A), Pdcd4(D418A), or Pdcd4(D235A,D418A). Together, these results indicate that not only binding to eIF4A but also prevention of eIF4A binding to the MA-3 domain of eIF4Gc contributes to the mechanism by which Pdcd4 inhibits translation.
Figures
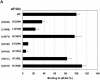
FIG. 1.
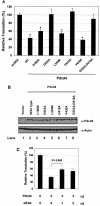
Both MA-3 domains of Pdcd4 are required for eIF4A-binding activity. (A) Structures of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. The numbers refer to the size (in amino acids) of eIF4G1, DAP-5/NAT1/p97, and Pdcd4 and to the location of the eIF4A-binding domain and the MA-3 domain (2, 17, 24). The MA-3 domains (gray box) in eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated schematically. The eIF4A-binding domains (strip box and arrows) in eIF4G1 are indicated schematically. (B) Partial deletion of N- or C-terminal MA-3 domain of Pdcd4 dramatically decreases the eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or deletion mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviation. (C) Protein expression level of wild-type and truncated Gal4 DNA-binding domain-Pdcd4. Cell lysates (15 μg) from transiently transfected empty vector (lane 1), wild-type (WT) Pdcd4 (lane 2), or truncated Pdcd4 (lanes 3 and 4) were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or actin antibody with visualization by chemiluminescent detection.
FIG. 1.
Both MA-3 domains of Pdcd4 are required for eIF4A-binding activity. (A) Structures of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. The numbers refer to the size (in amino acids) of eIF4G1, DAP-5/NAT1/p97, and Pdcd4 and to the location of the eIF4A-binding domain and the MA-3 domain (2, 17, 24). The MA-3 domains (gray box) in eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated schematically. The eIF4A-binding domains (strip box and arrows) in eIF4G1 are indicated schematically. (B) Partial deletion of N- or C-terminal MA-3 domain of Pdcd4 dramatically decreases the eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or deletion mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviation. (C) Protein expression level of wild-type and truncated Gal4 DNA-binding domain-Pdcd4. Cell lysates (15 μg) from transiently transfected empty vector (lane 1), wild-type (WT) Pdcd4 (lane 2), or truncated Pdcd4 (lanes 3 and 4) were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or actin antibody with visualization by chemiluminescent detection.
FIG. 1.
Both MA-3 domains of Pdcd4 are required for eIF4A-binding activity. (A) Structures of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. The numbers refer to the size (in amino acids) of eIF4G1, DAP-5/NAT1/p97, and Pdcd4 and to the location of the eIF4A-binding domain and the MA-3 domain (2, 17, 24). The MA-3 domains (gray box) in eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated schematically. The eIF4A-binding domains (strip box and arrows) in eIF4G1 are indicated schematically. (B) Partial deletion of N- or C-terminal MA-3 domain of Pdcd4 dramatically decreases the eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or deletion mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviation. (C) Protein expression level of wild-type and truncated Gal4 DNA-binding domain-Pdcd4. Cell lysates (15 μg) from transiently transfected empty vector (lane 1), wild-type (WT) Pdcd4 (lane 2), or truncated Pdcd4 (lanes 3 and 4) were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or actin antibody with visualization by chemiluminescent detection.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 2.
The MA-3 domain of DAP-5/NAT1/p97 is not functional for binding to eIF4A. (A) Alignment of the MA-3 domain of eIF4G1, DAP-5/NAT1/p97, and Pdcd4. Conserved (yellow box) and similar (red box) amino acid residues between eIF4G1, DAP-5/NAT1/p97, and Pdcd4 are indicated. The amino acids that are conserved between eIF4G1 and Pdcd4 but are different from DAP-5/NAT1/p97 are marked with asterisks. (B and C) Point mutation analyses of the C-terminal (B) and N-terminal (C) MA-3 domain of Pdcd4 for eIF4A-binding activity. Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or site-specific mutants) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into mouse JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. In panel C, the corresponding mutant in the C-terminal MA-3 domain is indicated in parentheses. (D) Similar expression of wild-type and mutant Gal4 DNA-binding domain-Pdcd4. RT101 cell lysates (10 μg) from transient transfection with wild-type (lane 1) and mutant (lanes 2 to 8) pCMV-BD-Pdcd4 expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (E) Pull-down assay of Pdcd4 with His-eIF4A. RT101 cell lysates from a transient transfection with Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific Pdcd4 mutant expression plasmids (lanes 3 to 6) were pulled down with His-eIF4A. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 or eIF4A antibody. (F) Coimmunoprecipitation of eIF4A with xpress-tagged Pdcd4. RT101 cell lysates from transient transfection with xpress-tagged Pdcd4 wild-type expression plasmid (lane 1), empty vector (lane 2), or site-specific xpress-tagged Pdcd4 mutant expression plasmids (lanes 3 to 8) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved by using a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 3.
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 3.
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 3.
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 3.
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
FIG. 3.
eIF4Gc has an MA-3 domain that is functional for eIF4A binding. (A) Plasmid pCMV-BD-eIF4Gc (50 ng) (wild type [WT] or site-specific mutant) was transiently transfected with pCMV-AD-eIF4A (400 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. (B) Similar expression of wild-type and mutant Gal4 DNA-binding domain-eIF4Gc. RT101 cell lysates (10 μg) from a transient transfection with wild type (lane 2) and site-specific mutants (lanes 3 to 6) pCMV-BD-eIF4Gc expression plasmids were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Gal4 DNA-binding domain antibody, with visualization by chemiluminescent detection. (C) Coimmunoprecipitation of eIF4A with xpress-tagged eIF4Gc. RT101 cell lysates from transient transfection with xpress-tagged eIF4Gc (lane 1), xpress-tagged eIF4GcD1259N (lane 2), or xpress-tagged eIF4GcE1329K (lane 3) expression plasmids were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody. (D) Plasmid pCMV-BD-Pdcd4 (50 ng) (wild type [WT] or point mutation mutants) or pCMV-BD-eIF4Gc (50 ng) was transiently transfected with pCMV-AD-eIF4A (50 ng) and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity with wild-type Pdcd4 was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as the means plus the standard deviations. An asterisk indicates a significant difference compared to the transfection with pCMV-BD-eIF4Gc, as determined by Student t test (P < 0.005). (E) Relative eIF4A-binding activity of eIF4Gc. RT101 cell lysates from a transient transfection with empty vector (lane 1) xpress-tagged Pdcd4 expression plasmid (lane 2) or xpress-tagged eIF4Gc expression plasmid (lane 3) were immunoprecipitated with mouse monoclonal xpress antibody. The bound proteins were resolved on a 10% Bis-Tris NuPage gel, transferred to nitrocellulose, and subjected to immunoblotting with eIF4A or xpress antibody.
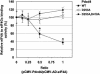
FIG. 4.
Pdcd4 prevents eIF4A binding to eIF4Gc. Increasing amounts (0, 50, 100, 200, and 400 ng) of pCMV-NLS-Pdcd4 (or pCMV-NLS-Pdcd4D235A or pCMV-NLS-Pdcd4D253A,D418A) were transiently transfected with pCMV-AD-eIF4A (400 ng), pCMV-BD-eIF4Gc (50 ng), and Gal4-luciferase reporter DNA (25 ng) into JB6 RT101 cells. After 48 h, cells were lysed and the luciferase activity was measured. The luciferase activity from 0 ng of pCMV-BD-Pdcd4, pCMV-BD-Pdcd4D253A, or pCMV-BD-Pdcd4D253A,D418A was designated as 100%. These experiments were repeated two times with six independent transfections, and representative data are shown. The results are expressed as means ± the standard deviations. Asterisks indicate significant differences compared to the transfection with 200 or 400 ng of pCMV-BD-Pdcd4D235A, as determined by using the Student t test (P < 0.001).
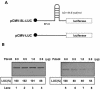
FIG. 5.
Pdcd4 preferentially inhibits translation of 5′UTR structured mRNA in vitro. (A) Structures of 5′UTR stem-loop structured luciferase (pCMV-SL-LUC) and nonstructured luciferase (pCMV-LUC); (B) in vitro translation of 5′UTR structured and nonstructured luciferase. Rabbit reticulocyte lysate was preincubated with increasing amounts of Pdcd4 (0 to 2 μg) for 5 min at 30°C prior to the addition of the nonstructured luciferase mRNA (0.2 μg) (lanes 1 to 4) or 5′UTR structured luciferase mRNA (0.2 μg) (lanes 5 to 8). The translation was performed in a total volume of 20 μl as described in Materials and Methods. The band intensity was determined with a PhosphorImager. The value obtained for nonstructured or structured luciferase mRNA with 0 μg of Pdcd4 was designated as 100%.
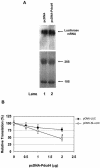
FIG. 6.
Pdcd4 preferentially inhibits translation of 5′UTR structured mRNA in transfected cells. (A) Northern blotting of luciferase mRNA. Empty vector (10 μg) (lane 1) or Pdcd4 expression plasmid (10 μg) (lane 2) was transiently transfected with pCMV-SL-LUC (1 μg) into RT101 cells. After 48 h, the total RNA was isolated, separated on a 1% formaldehyde agarose gel, transferred to a nylon membrane, and visualized by hybridization with 32P-labeled luciferase cDNA. (B) Translation of structured and nonstructured mRNA in transfected cells. The plasmid pcDNA-Pdcd4 (0 to 2 μg) was transiently transfected with pCMV-LUC (0.2.μg) (•) or pCMV-SL-LUC (0.2 μg) (○) into RT101 cells. The total DNA was maintained at 2.2 μg by adding pcDNA3.1+ vector DNA. After transfection, cells were serum starved (0.2% FBS) for 24 h and then incubated with complete medium (4% FBS) for an additional 24 h. The luciferase activity from the cells with 0 μg of pCMV-LUC or pCMV-SL-LUC was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as means ± standard deviations. An asterisk indicates significant differences compared to the transfection with 2 μg of pCMV-LUC plasmid, as determined by using the Student t test (P < 0.001).
FIG. 7.
Pdcd4 mutants that are defective for binding to eIF4A fail to inhibit translation. (A) Portions (2 μg) of wild-type Pdcd4 expression plasmid (WT), empty vector (pcDNA), or site-specific Pdcd4 mutant expression plasmids (as indicated) were transiently transfected with pCMV-SL-LUC (0.2 μg) into RT101 cells. The total DNA was maintained at 2.2 μg by adding pcDNA3.1+ vector DNA. (B) Similar expression level of wild-type and mutant Pdcd4. RT101 cell lysates (10 μg) from transient transfection with empty vector (lane 1), wild-type Pdcd4 (lane 2), and Pdcd4 mutant expression plasmids (lanes 3 to 8) were separated on 10% Bis-Tris NuPage gels, transferred to nitrocellulose, and subjected to immunoblotting with Pdcd4 antibody, with visualization by chemiluminescent detection (C) Partial reversal by eIF4A of Pdcd4 inhibition of translation. Portions (2 μg) of Pdcd4 expression plasmid and eIF4A expression plasmid (1 or 2 μg) were cotransfected with pCMV-SL-LUC (0.2 μg) into RT101 cells. The total DNA was maintained at 4.2 μg by adding pcDNA3.1+ vector DNA. After transfection, cells were serum starved (0.2% fetal bovine serum) for 24 h and then incubated with complete medium (4% FBS) for an additional 24 h. The luciferase activity from the cells transfected with empty vector was designated as 100%. These experiments were repeated three times with five independent transfections, and representative data are shown. The results are expressed as means plus the standard deviations.
FIG. 8.
Model for Pdcd4 binding to eIF4A. (A) Two MA-3 domains (gray box) of Pdcd4 bind to two compacted domains of eIF4A within the Pdcd4-binding region (black) to achieve a maximal binding activity. (B) When N- or C-terminal MA-3 domain of Pdcd4 is mutated and is defective in binding to the compacted domain of eIF4A, the mutated Pdcd4 only contains one intact MA-3 domain, which is able to bind to the compacted domain of eIF4A. In this case, the mutated Pdcd4 has a very weak eIF4A-binding activity.
Similar articles
- The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation.
Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH. Yang HS, et al. Mol Cell Biol. 2003 Jan;23(1):26-37. doi: 10.1128/MCB.23.1.26-37.2003. Mol Cell Biol. 2003. PMID: 12482958 Free PMC article. - Mutational analysis of the DEAD-box RNA helicase eIF4AII characterizes its interaction with transformation suppressor Pdcd4 and eIF4GI.
Zakowicz H, Yang HS, Stark C, Wlodawer A, Laronde-Leblanc N, Colburn NH. Zakowicz H, et al. RNA. 2005 Mar;11(3):261-74. doi: 10.1261/rna.7191905. Epub 2005 Jan 20. RNA. 2005. PMID: 15661843 Free PMC article. - PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains.
Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, Wagner G. Suzuki C, et al. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3274-9. doi: 10.1073/pnas.0712235105. Epub 2008 Feb 22. Proc Natl Acad Sci U S A. 2008. PMID: 18296639 Free PMC article. - Eukaryotic initiation factor 4A (eIF4A) during viral infections.
Montero H, Pérez-Gil G, Sampieri CL. Montero H, et al. Virus Genes. 2019 Jun;55(3):267-273. doi: 10.1007/s11262-019-01641-7. Epub 2019 Feb 22. Virus Genes. 2019. PMID: 30796742 Free PMC article. Review. - Roles of helicases in translation initiation: a mechanistic view.
Marintchev A. Marintchev A. Biochim Biophys Acta. 2013 Aug;1829(8):799-809. doi: 10.1016/j.bbagrm.2013.01.005. Epub 2013 Jan 19. Biochim Biophys Acta. 2013. PMID: 23337854 Free PMC article. Review.
Cited by
- The Impact of Pdcd4, a Translation Inhibitor, on Drug Resistance.
Wang Q, Yang HS. Wang Q, et al. Pharmaceuticals (Basel). 2024 Oct 19;17(10):1396. doi: 10.3390/ph17101396. Pharmaceuticals (Basel). 2024. PMID: 39459035 Free PMC article. Review. - The ezrin metastatic phenotype is associated with the initiation of protein translation.
Briggs JW, Ren L, Nguyen R, Chakrabarti K, Cassavaugh J, Rahim S, Bulut G, Zhou M, Veenstra TD, Chen Q, Wei JS, Khan J, Uren A, Khanna C. Briggs JW, et al. Neoplasia. 2012 Apr;14(4):297-310. doi: 10.1593/neo.11518. Neoplasia. 2012. PMID: 22577345 Free PMC article. - The unique evolution of the programmed cell death 4 protein in plants.
Cheng S, Liu R, Gallie DR. Cheng S, et al. BMC Evol Biol. 2013 Sep 16;13:199. doi: 10.1186/1471-2148-13-199. BMC Evol Biol. 2013. PMID: 24041411 Free PMC article. - IGF-1R inhibition induces MEK phosphorylation to promote survival in colon carcinomas.
Wang Q, Zhang Y, Zhu J, Zheng H, Chen S, Chen L, Yang HS. Wang Q, et al. Signal Transduct Target Ther. 2020 Aug 26;5(1):153. doi: 10.1038/s41392-020-0204-0. Signal Transduct Target Ther. 2020. PMID: 32843616 Free PMC article. - Cellular adaptation to nutrient deprivation: crosstalk between the mTORC1 and eIF2α signaling pathways and implications for autophagy.
Wengrod JC, Gardner LB. Wengrod JC, et al. Cell Cycle. 2015;14(16):2571-7. doi: 10.1080/15384101.2015.1056947. Cell Cycle. 2015. PMID: 26039820 Free PMC article.
References
- Abramson, R. D., T. E. Dever, T. G. Lawson, B. K. Ray, R. E. Thach, and W. C. Merrick. 1987. The ATP-dependent interaction of eukaryotic initiation factors with mRNA. J. Biol. Chem. 262:3826-3832. - PubMed
- Azzoni, L., O. Zatsepina, B. Abede, I. M. Bennett, P. Kanakaraj, and B. Perussia. 1998. Differential transcriptional regulation of CD161 and a novel gene, 197/15a, by IL-2, IL-15, and IL-12 in NK and T cells. J. Immunology 161:3493-3500. - PubMed
- Bohm, M., K. Sawicka, J. P. Siebrasse, A. Brehmer-Fastnacht, R. Peters, and K. H. Klempnauer. 2003. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene 22:4905-4910. - PubMed
- Brown, E. J., and S. L. Schreiber. 1996. A signaling pathway to translational control. Cell 86:517-520. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous