Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I - PubMed (original) (raw)
Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I
Gary A Wayman et al. J Neurosci. 2004.
Abstract
Calcium and calmodulin (CaM) are important signaling molecules that regulate axonal or dendritic extension and branching. The Ca2+-dependent stimulation of neurite elongation has generally been assumed to be mediated by CaM-kinase II (CaMKII), although other members of the CaMK family are highly expressed in developing neurons. We have examined this assumption using a combination of dominant-negative CaMKs (dnCaMKs) and other specific CaMK inhibitors. Here we report that inhibition of cytosolic CaMKI, but not CaMKII or nuclear CaMKIV, dramatically decreases axonal outgrowth and branching in cultured neonatal hippocampal and postnatal cerebellar granule neurons. CaMKI is found throughout the cell cytosol, including the growth cone. Growth cones of neurons expressing dnCaMI or dnCaMKK, the upstream activator of CaMKI, exhibit collapsed morphology with a prominent reduction in lamellipodia. Live-cell imaging confirms that these morphological changes are associated with a dramatic decrease in growth cone motility. Treatment of neurons with 1,8-naphthoylene benzimidazole-3-carboxylic acid (STO-609), an inhibitor of CaMKK, causes a similar change in morphology and reduction in growth cone motility, and this inhibition can be rescued by transfection with an STO-609-insensitive mutant of CaMKK or by transfection with constitutively active CaMKI. These results identify CaMKI as a positive transducer of growth cone motility and axon outgrowth and provide a new physiological role for the CaMKK-CaMKI pathway.
Figures
Figure 1.
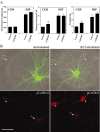
Neurite growth in hippocampal and cerebellar granule cells is not suppressed by inhibition of CaMKII. A, High-density cultures of rat hippocampal (HIP) and cerebellar granule cell neurons (CER) were transfected on day 2 with plasmid encoding soluble EGFP alone (control) or in combination with the specific CaMKII inhibitor, CaMKIIN. Neurons were cultured an additional 2 d and then fixed and imaged. Results show total neurite length, number of primary neurites, and the number of neurite terminals(reflecting neurite number and branching), based on the analysis of 30 cells in each condition (means ± SD). *p values of <0.01 (t test). B, CaMKIIN inhibits CaMKII activity in hippocampal neurons. Low-density hippocampal cultures were transfected on day 5 with plasmid encoding EGFP-CaMKIIN. The neurons were cultured an additional 9 d and then stained with phospho-dependent Ab for Thr286 in CaMKII (pCaMKII), either with (right panels) or without (left panels) previous stimulation with 90 m
m
KCl (5 min). After KCl stimulation, nontransfected neurons (arrowheads) show enhanced staining for activated CaMKII (autophosphorylated at Thr286) throughout their cell bodies and processes (bottom panel). EGFP-CaMKIIN-expressing neurons (arrows) show no increase in anti-phospho-CaMKII staining over levels seen in unstimulated cells, demonstrating that overexpression of CaMKIIN inhibited activation of endogenous CaMKII. Scale bars: A,10 μm; B,50 μm.
Figure 2.
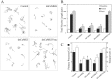
Hippocampal neurite growth is suppressed by inhibition of the CaMKK–CaMKI pathway but not by inhibition of nuclear CaMKIV. High-density cultures of rat hippocampal neurons were transfected on day 2 with plasmid encoding soluble EGFP alone (control) or in combination with dnCaKK, dnCaMKI, dnCaMKIV, or dnCaMKIVnuc. Neurons were cultured an additional 2 d and then fixed and imaged. Camera lucida drawings (A) of control and CaMK-expressing neurons are shown. Quantification of the effects of inhibiting CaMKs K, I, or IV are shown in B and C. Axons and dendrites were identified by morphology, and neurite growth was quantified as in Figure 1 (30 cells per condition). *p values of <0.01 (t test).
Figure 3.
Cerebellar granule cell neurite growth is suppressed by inhibition of the CaMKK–CaMKI pathway. High-density cultures of rat cerebellar granule cell neurons were transfected on day 2 with plasmid encoding soluble EGFP alone (control) or in combination with dnCaKK, dnCaMKI, or dnCaMKIVnuc. Neurons were cultured an additional 2 d and then fixed and imaged. Camera lucida drawings (A) of control and CaMK-expressing neurons are shown. Quantification of the effects of inhibiting CaMKs K, I, or IV are shown in B and C. Axons and dendrites were identified by morphology, and neurite growth was quantified as in Figure 1 (30 cells per condition). *p values of <0.01 (t test).
Figure 4.
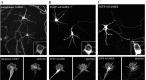
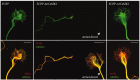
Localization of CaMKI and CaMKK in cultured hippocampal neurons. A, Hippocampal neurons (4 DIV) were immunostained for endogenous CaMKI. B, C, Low-density hippocampal cultures were transfected on day 3 with plasmid encoding EGFP-wtCaMKI (B) or EGFP-wtCaMKK (C). After 16 hr, neurons were fixed and stained with fluorescently labeled phalloidin and imaged by confocal microscopy. Endogenous CaMKI, EGFP-wtCaMKI, and EGFP-wtCaMKK are excluded from the nucleus (insets) but distribute throughout the processes, including the axonal growth cones (bottom panels) extending into the lamellipodia and some, but not all, filopodia. Scale bars: top, 50 μm; bottom, 10 μm.
Figure 5.
Inhibition of CaMKI results in axonal growth cone collapse. Low-density hippocampal neurons were transfected with constructs encoding dn- and wtCaMKI on day 3 and then fixed 8 hr later. Cells were immunolabeled to stain tyrosinatedα-tubulin using Cy5-labeled secondary antibodies (pseudocolored green in bottom panel) and stained with tetramethylrhodamine isothiocyanate-labeled phalloidin to visualize filamentous actin (pseudocolored red in bottom panel). In neurons expressing only EGFP(left panel), the peripheral domain of the growth cone is dominated by actin(red), where as the central domain contains both actin and stabilized microtubules (green; yellow in merged image). Expression of EGFP-dnCaMKI (middle panel, top) dramatically reduced growth cone size (middle panel, left) compared with the growth cone of a neighboring untransfected neuron (middle panel, right). Both peripheral and central domains of the growth cone were essentially eliminated by expression of dnCaMKI. Expression of wtCaMKI had little effect on growth cone morphology (right panel). Scale bar, 10 μm.
Figure 6.
Inhibition of CaMKI suppresses axonal growth cone motility. Axonal growth cone motility was imaged in low-density cultures of 3-d-old hippocampal neurons 8 hr (A_–_C, top panels) or 20 hr (C, bottom panels) after transfection with soluble EGFP (A), EGFP-wtCaMKI (B), or EGFP-dnCaMKI (C). Fluorescence images were acquired every 5 sec over a period of 5 min. Individual images taken at 25 sec intervals are shown outlined in yellow. To graphically represent motility (right panel), the area of the growth cone exhibiting motility (occupied by the growth cone in some but not all images) is shown in white. Green arrows highlight areas of growth cone advance; red arrows highlight areas of growth cone retraction. In neurons expressing dnCaMKI, the area explored by a growth cone during 5 min of recording was dramatically reduced compared with the growth cones of control cells expressing EGFP alone or EGFP-wtCaMKI.
Figure 7.
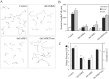
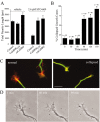
Inhibition of CaMKK by STO-609 inhibits neurite outgrowth and causes axonal growth cone collapse. A, High-density cultures of rat hippocampal neurons were transfected on day 2 with plasmid encoding soluble EGFP alone (Control) or in combination with STO-609-insensitive CaMKKL233F or caCaMKI. Neurons were cultured for 12 hr to allow for expression of the transfected protein and then treated without (i.e., vehicle) or with 2.6 μ
m
STO-609 for 2 d. Neurons were then fixed and imaged. Quantification of total neurite length is shown for 30 cells per condition. Statistical analysis compares neurite length of kinase-expressing (CaMKKL233F or caCaMKI) versus control cells in the absence or presence of STO-609. *p values of <0.01 (t test). B, C, Inhibition of CaMKK by STO-609 results in growth cone collapse. Low-density cultures of 3-d-old hippocampal neurons were treated with 2.6 μ
m
of STO-609 and then fixed and stained for filamentous actin (phalloidin; red) and microtubules (green). Randomly selected axonal growth cones (80–100 per condition) were scored as normal (C, left panel) or collapsed (C, right panel) by an investigator without knowledge of the treatment conditions. The bar graph (B) summarizes the percentage of growth cones that were collapsed as a function of time after addition of STO-609 (± SE of percentage). After 90 min in STO-609 the percentage of collapsed growth cones quadrupled. Scale bar: C,10 μm. D, Inhibition of CaMKK by STO-609 suppresses growth cone motility. At 5, 60, or 90 min after addition of 2.6 μ
m
STO-609, axonal growth cone motility was assessed in an individual neuron by acquiring phase-contrast images every 5 sec for a period of 5 min. Single images of these time-lapse recordings show a change from a spread growth cone morphology with lamellar veils extending between filopodia (left) to a reduced growth cone size at 60 min (middle) and 90 min (right), with fewer and smaller lamellar extensions. The time-lapse movie available as supplemental material shows that STO-609 treatment markedly inhibited the motility of this growth cone after 60 and 90 min of treatment. Growth cones of untreated sister cultures showed no reduction of growth cone motility over a period of 120 min (data not shown).
Similar articles
- Analysis of CaM-kinase signaling in cells.
Wayman GA, Tokumitsu H, Davare MA, Soderling TR. Wayman GA, et al. Cell Calcium. 2011 Jul;50(1):1-8. doi: 10.1016/j.ceca.2011.02.007. Epub 2011 Apr 29. Cell Calcium. 2011. PMID: 21529938 Free PMC article. Review. - Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation.
Schmitt JM, Guire ES, Saneyoshi T, Soderling TR. Schmitt JM, et al. J Neurosci. 2005 Feb 2;25(5):1281-90. doi: 10.1523/JNEUROSCI.4086-04.2005. J Neurosci. 2005. PMID: 15689566 Free PMC article. - Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in spiral ganglion neurons.
Hansen MR, Bok J, Devaiah AK, Zha XM, Green SH. Hansen MR, et al. J Neurosci Res. 2003 Apr 15;72(2):169-84. doi: 10.1002/jnr.10551. J Neurosci Res. 2003. PMID: 12671991 - Calcium activation of ERK mediated by calmodulin kinase I.
Schmitt JM, Wayman GA, Nozaki N, Soderling TR. Schmitt JM, et al. J Biol Chem. 2004 Jun 4;279(23):24064-72. doi: 10.1074/jbc.M401501200. Epub 2004 Mar 29. J Biol Chem. 2004. PMID: 15150258 - Research advances on CaMKs-mediated neurodevelopmental injury.
Kong L, Yang J, Yang H, Xu B, Yang T, Liu W. Kong L, et al. Arch Toxicol. 2024 Dec;98(12):3933-3947. doi: 10.1007/s00204-024-03865-5. Epub 2024 Sep 18. Arch Toxicol. 2024. PMID: 39292234 Review.
Cited by
- CaMKIV Signaling Is Not Essential for the Maintenance of Intrinsic or Synaptic Properties in Mouse Visual Cortex.
Trojanowski NF, Turrigiano GG. Trojanowski NF, et al. eNeuro. 2021 Jul 6;8(4):ENEURO.0135-21.2021. doi: 10.1523/ENEURO.0135-21.2021. Print 2021 Jul-Aug. eNeuro. 2021. PMID: 34001638 Free PMC article. - Cultures of cerebellar granule neurons.
Bilimoria PM, Bonni A. Bilimoria PM, et al. CSH Protoc. 2008 Dec 1;2008:pdb.prot5107. doi: 10.1101/pdb.prot5107. CSH Protoc. 2008. PMID: 21356753 Free PMC article. - Analysis of CaM-kinase signaling in cells.
Wayman GA, Tokumitsu H, Davare MA, Soderling TR. Wayman GA, et al. Cell Calcium. 2011 Jul;50(1):1-8. doi: 10.1016/j.ceca.2011.02.007. Epub 2011 Apr 29. Cell Calcium. 2011. PMID: 21529938 Free PMC article. Review. - Signaling mechanisms in cortical axon growth, guidance, and branching.
Kalil K, Li L, Hutchins BI. Kalil K, et al. Front Neuroanat. 2011 Sep 28;5:62. doi: 10.3389/fnana.2011.00062. eCollection 2011. Front Neuroanat. 2011. PMID: 22046148 Free PMC article. - AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization.
Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY. Amato S, et al. Science. 2011 Apr 8;332(6026):247-51. doi: 10.1126/science.1201678. Epub 2011 Mar 24. Science. 2011. PMID: 21436401 Free PMC article.
References
- Baouz S, Jacquet E, Accorsi K, Hountondji C, Balestrini M, Zippel R, Sturani E, Parmeggiani A (2001) Sites of phosphorylation by protein kinase A in CDC25Mm/GRF1, a guanine nucleotide exchange factor for Ras. J Biol Chem 276: 1742–1749. - PubMed
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042–2045. - PubMed
- Brewer GJ (1997) Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods 71: 143–155. - PubMed
- Carpenter CL, Cantley LC (1996) Phosphoinositide kinases. Curr Opin Cell Biol 8: 153–158. - PubMed
- Chadborn N, Eickholt B, Doherty P, Bolsover S (2002) Direct measurement of local raised subplasmalemmal calcium concentrations in growth cones advancing on an N-cadherin substrate. Eur J Neurosci 15: 1891–1898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS017112/NS/NINDS NIH HHS/United States
- NS17112/NS/NINDS NIH HHS/United States
- DK44239/DK/NIDDK NIH HHS/United States
- NS27037/NS/NINDS NIH HHS/United States
- P01 DK044239/DK/NIDDK NIH HHS/United States
- R01 NS027037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous