F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis - PubMed (original) (raw)
F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis
Martin van der Laan et al. J Cell Biol. 2004.
Abstract
The Escherichia coli YidC protein belongs to the Oxa1 family of membrane proteins that have been suggested to facilitate the insertion and assembly of membrane proteins either in cooperation with the Sec translocase or as a separate entity. Recently, we have shown that depletion of YidC causes a specific defect in the functional assembly of F1F0 ATP synthase and cytochrome o oxidase. We now demonstrate that the insertion of in vitro-synthesized F1F0 ATP synthase subunit c (F0c) into inner membrane vesicles requires YidC. Insertion is independent of the proton motive force, and proteoliposomes containing only YidC catalyze the membrane insertion of F0c in its native transmembrane topology whereupon it assembles into large oligomers. Co-reconstituted SecYEG has no significant effect on the insertion efficiency. Remarkably, signal recognition particle and its membrane-bound receptor FtsY are not required for the membrane insertion of F0c. In conclusion, a novel membrane protein insertion pathway in E. coli is described in which YidC plays an exclusive role.
Figures
Figure 1.
In vitro–synthesized F 0 c inserts into E . coli inner membrane vesicles. (A) Membrane topology model of F0c based on the NMR solution structure (1A91.pdb). Arrows indicate the positions of the introduced cysteine mutations for topology analysis. (B) In vitro–synthesized F0c inserts into E. coli wild-type IMVs. Translation reactions were performed in the presence (lanes 1–3) or absence (lanes 4 and 5) of 5-μg IMVs. Subsequently, the translation reaction samples were treated with 0.4 mg/ml proteinase K (lanes 2, 3, and 5) in the presence (lane 3) or absence (lanes 1, 2, 4, and 5) of 1% (vol/vol) Triton X-100. In the presence of Triton X-100, a proteolytic fragment of F0c is formed (asterisk). Where indicated, 20% standards of the translation reactions are shown. (C) A membrane protein is required for the membrane insertion of F0c. IMVs were pretreated in the presence (lanes 1 and 3) or absence (lanes 2 and 4) of proteinase K as described in the Materials and methods section. (D) The PMF is not required for the membrane insertion of F0c. Insertion reactions were performed in the absence (lanes 1 and 3) or in the presence (lanes 2 and 4) of 1 μM valinomycin (val) and 1 μM nigericin (nig). Insertion efficiencies were calculated from three independent experiments.
Figure 2.
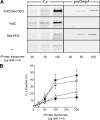
YidC is required for the membrane insertion of F 0 c. (A) YidC can be efficiently depleted from E. coli JS7131 IMVs. Cells were grown as described in the Materials and methods section in the presence of 0.2% arabinose (lane 1) or 0.2% glucose (lane 2), and the amounts of YidC in IMVs were monitored by Western blotting. YidC-depleted IMVs exhibit reduced proOmpA translocation (B, lanes 1–3) and FtsQ insertion activities (C, lanes 1–4). The same extents of reduction are observed when the reactions are performed with wild-type IMVs in the presence of 1 μM valinomycin (val) and 1 μM nigericin (nig) to uncouple the PMF (B, lane 4; C, lanes 5 and 6). Post-translational proOmpA translocation as well as cotranslational FtsQ insertion reactions contained 25-μg IMVs. Efficiencies given in B and C were calculated from two independent experiments. (D) YidC depletion blocks membrane insertion of F0c. F0c insertion reactions were performed as described in the legend to Fig. 1. Efficiencies given in D were calculated from three independent experiments.
Figure 3.
YidC alone is sufficient to catalyze the membrane insertion of F0c into proteoliposomes. (A) Co-translational F0c insertion as well as post-translational proOmpA translocation reactions were performed in the presence of the indicated amounts of (proteo-)liposomes reconstituted with YidC and SecYEG (YidC/SecYEG), YidC alone, SecYEG alone, or without any proteins (−). (B) F0c insertion efficiency at different (proteo-)liposome concentrations was quantified from the amount of protease-protected material in the presence of SecYEG/YidC (▪), YidC (○), SecYEG (□), or protein-free (•) liposomes. The amount of F0c synthesized was comparable in all reactions (not depicted) and was set as 100%. All data points shown in B are averages from five independent experiments in which proteoliposomes from three independent reconstitutions were used. Purified SecYEG and YidC were both used at a protein/lipid ratio of 1:100 (wt/wt).
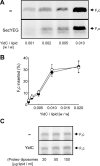
Figure 4.
SecYEG is not required for F0c membrane insertion. (A) Reactions were performed as described in the legend of Fig. 3 in the presence of 100 μg lipid/ml proteoliposomes. For the reconstitutions, YidC was used at the indicated protein-to-lipid ratios together with (SecYEG) or without (−) a fixed amount of SecYEG (protein/lipid ratio of 1:100 [wt/wt]). (B) F0c insertion efficiencies were quantified from the amount of protease-protected material in the presence (•) or absence (○) of SecYEG. Synthesis of F0c was comparable in all reactions (not depicted). All data points shown in B are averages from three independent experiments. Proteoliposomes from two independent reconstitutions were used. When added after translation has been stopped with chloramphenicol, YidC proteoliposomes do not stimulate membrane insertion of F0c (C). Translation reactions were performed in the absence of membranes. Reactions were stopped by the addition of 25 μg/ml chloramphenicol. After 5 min, indicated amounts of YidC proteoliposomes or protein-free liposomes were added, and reaction mixtures were incubated for 20 min at 37°C.
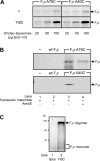
Figure 5.
Membrane insertion of F0c does not require the SRP pathway or SecA. (A) Translation lysate was immunodepleted from either FtsY or SecA, and F0c insertion reactions were performed in the presence of YidC proteoliposomes (YidC PL, 100 μg lipid/ml; protein/lipid ratio 1:100). (B) A Western blot with α-FtsY antiserum using 20 μg wild-type (lane 1) or FtsY-depleted (lane 2) lysate. (C) Depletion of FtsY from the lysate inhibits the FtsQ insertion into SecYEG proteoliposomes. Reactions were performed with FtsY-depleted (lanes 1 and 2) or wild-type (lanes 3 and 4) lysate in the presence of Na+-loaded SecYEG proteoliposomes (200 μg lipid/ml) and 1 μM valinomycin to generate a transmembrane electrical potential. (D) Ffh can be efficiently depleted in vivo in E. coli strain HDB51. A Western blot performed with 25 μg HDB51 wild-type (lane 1) or Ffh-depleted (lane 2) lysate is shown. (E) Ffh depletion has no effect on F0c insertion into IMVs (lane 1) or proteoliposomes containing YidC (lane 2) or YidC together with SecYEG (lane 3). Reactions were performed with wild-type or Ffh-depleted HDB51 lysate in the presence of 5-μg IMVs or 100 μg lipid/ml proteoliposome. Purified YidC and SecYEG were reconstituted at a protein/lipid ratio of 1:100. (F) Ffh depletion inhibits membrane insertion of FtsQ. Reactions were performed with Ffh-depleted (lanes 1 and 2) and wild-type (lanes 3 and 4) HDB51 lysate in the presence of 25-μg IMVs or 200 μg lipid/ml Na+-loaded SecYEG proteoliposome. FtsQ and F0c insertion efficiencies were calculated from two and three independent experiments, respectively.
Figure 6.
F0c inserted into YidC proteoliposomes has acquired its native transmembrane topology. (A) F0c A79C and F0c A40C insert into YidC proteoliposomes comparable to the wild-type protein. Reactions were performed as described in the legend to Fig. 3 in the presence of the indicated amounts of (proteo-)liposomes. (B) For cysteine accessibility experiments, wild-type F0c and both single-cysteine derivatives were synthesized and inserted into YidC proteoliposomes in nonradioactive translation reactions. Subsequent labeling was performed with 1 mM fluorescein maleimide (lanes 1–4) as described in the Materials and methods section. A preincubation with 0.5 mM AMdiS was performed where indicated (lane 4). To control specificity of labeling, the same reactions were performed in the absence of mRNA (lane 1) or with the cysteine-free wild-type F0c (lane 2). (C) In vitro–synthesized F0c assembles into large oligomeric complexes after insertion into YidC proteoliposomes. Reactions were performed in the presence of protein-free liposomes (lane 1) or YidC proteoliposomes (lane 2) as described in the Materials and methods section, and the high molecular mass oligomers were analyzed by Blue Native PAGE. On this gel system, monomeric F0c (in the presence of 1% SDS) migrates as a very diffuse polypeptide band (not depicted).
Similar articles
- YidC is strictly required for membrane insertion of subunits a and c of the F(1)F(0)ATP synthase and SecE of the SecYEG translocase.
Yi L, Jiang F, Chen M, Cain B, Bolhuis A, Dalbey RE. Yi L, et al. Biochemistry. 2003 Sep 9;42(35):10537-44. doi: 10.1021/bi034309h. Biochemistry. 2003. PMID: 12950181 - F(1)F(0) ATP synthase subunit c is targeted by the SRP to YidC in the E. coli inner membrane.
van Bloois E, Jan Haan G, de Gier JW, Oudega B, Luirink J. van Bloois E, et al. FEBS Lett. 2004 Oct 8;576(1-2):97-100. doi: 10.1016/j.febslet.2004.08.069. FEBS Lett. 2004. PMID: 15474018 - Subunit a of the F(1)F(0) ATP synthase requires YidC and SecYEG for membrane insertion.
Kol S, Majczak W, Heerlien R, van der Berg JP, Nouwen N, Driessen AJ. Kol S, et al. J Mol Biol. 2009 Jul 31;390(5):893-901. doi: 10.1016/j.jmb.2009.05.074. Epub 2009 Jun 1. J Mol Biol. 2009. PMID: 19497329 - Mechanisms of YidC-mediated insertion and assembly of multimeric membrane protein complexes.
Kol S, Nouwen N, Driessen AJ. Kol S, et al. J Biol Chem. 2008 Nov 14;283(46):31269-73. doi: 10.1074/jbc.R800029200. Epub 2008 Jul 25. J Biol Chem. 2008. PMID: 18658156 Review. - The ribosome and YidC. New insights into the biogenesis of Escherichia coli inner membrane proteins.
de Gier JW, Luirink J. de Gier JW, et al. EMBO Rep. 2003 Oct;4(10):939-43. doi: 10.1038/sj.embor.embor921. EMBO Rep. 2003. PMID: 14528263 Free PMC article. Review.
Cited by
- Global change of gene expression and cell physiology in YidC-depleted Escherichia coli.
Wang P, Kuhn A, Dalbey RE. Wang P, et al. J Bacteriol. 2010 Apr;192(8):2193-209. doi: 10.1128/JB.00484-09. Epub 2010 Jan 8. J Bacteriol. 2010. PMID: 20061485 Free PMC article. - Constant c10 ring stoichiometry in the Escherichia coli ATP synthase analyzed by cross-linking.
Ballhausen B, Altendorf K, Deckers-Hebestreit G. Ballhausen B, et al. J Bacteriol. 2009 Apr;191(7):2400-4. doi: 10.1128/JB.01390-08. Epub 2009 Jan 30. J Bacteriol. 2009. PMID: 19181809 Free PMC article. - The Cellular Mechanisms that Ensure an Efficient Secretion in Streptomyces.
Gullón S, Mellado RP. Gullón S, et al. Antibiotics (Basel). 2018 Apr 14;7(2):33. doi: 10.3390/antibiotics7020033. Antibiotics (Basel). 2018. PMID: 29661993 Free PMC article. Review. - The conserved third transmembrane segment of YidC contacts nascent Escherichia coli inner membrane proteins.
Yu Z, Koningstein G, Pop A, Luirink J. Yu Z, et al. J Biol Chem. 2008 Dec 12;283(50):34635-42. doi: 10.1074/jbc.M804344200. Epub 2008 Oct 6. J Biol Chem. 2008. PMID: 18840604 Free PMC article. - Targeting and Insertion of Membrane Proteins.
Kuhn A, Koch HG, Dalbey RE. Kuhn A, et al. EcoSal Plus. 2017 Mar;7(2):10.1128/ecosalplus.ESP-0012-2016. doi: 10.1128/ecosalplus.ESP-0012-2016. EcoSal Plus. 2017. PMID: 28276312 Free PMC article. Review.
References
- Ackerman, S.H. 2002. Atp11p and Atp12p are chaperones for F1-ATPase biogenesis in mitochondria. Biochim. Biophys. Acta. 1555:101–105. - PubMed
- Ackerman, S.H., and A. Tzagoloff. 1990. ATP10, a yeast nuclear gene required for the assembly of the mitochondrial F1F0 complex. J. Biol. Chem. 265:9952–9959. - PubMed
- Altamura, N., N. Capitanio, N. Bonnefoy, S. Papa, and G. Dujardin. 1996. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase. FEBS Lett. 382:111–115. - PubMed
- Arechaga, I., P.J.G. Butler, and J.E. Walker. 2002. Self-assembly of ATP synthase subunit c rings. FEBS Lett. 515:189–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases