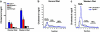Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease - PubMed (original) (raw)
Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease
Tamar Aprahamian et al. J Exp Med. 2004.
Abstract
To clarify the link between autoimmune disease and hypercholesterolemia, we created the gld.apoE(-/-) mouse as a model of accelerated atherosclerosis. Atherosclerotic lesion area was significantly increased in gld.apoE(-/-) mice compared with apoE(-/-) mice. gld.apoE(-/-) mice also displayed increases in lymphadenopathy, splenomegaly, and autoantibodies compared with gld mice, and these effects were exacerbated by high cholesterol diet. gld.apoE(-/-) mice exhibited higher levels of apoptotic cells, yet a reduced frequency of engulfed apoptotic nuclei within macrophages. Infusion of lysophosphatidylcholine, a component of oxidized low density lipoprotein, markedly decreased apoptotic cell clearance in gld mice, indicating that hypercholesterolemia promotes autoimmune disease in this background. These data suggest that defects in apoptotic cell clearance promote synergy between atherosclerotic and autoimmune diseases.
Figures
Figure 1.
Atherosclerotic lesions in the aortae of gld.apoE −/− and apoE −/− mice are visualized by Oil red O staining. The increase in atherosclerotic lesion area of the gld.apoE −/−, compared with the apoE −/−, is macroscopically visible on (a) Western diet, as well as (b) normal diet. The g_ld_ and wild-type mice showed no visible lesions (not depicted). (c) Quantification of Oil red O staining area revealed a statistically significant increase in lesion size on Western diet of the gld.apoE −/− (n = 26) compared with the apoE −/− (n = 27), and normal diet gld.apoE −/− (n = 11) compared with the apoE −/− (n = 8) (*, P < 0.001 vs. apoE −/−).
Figure 2.
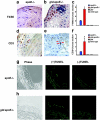
Vessel wall inflammation is increased in gld.apoE −/− mice compared with apoE −/− mice. Representative sections of atherosclerotic lesions from the aortic arch of the gld.apoE −/− and apoE −/− mice, maintained on a Western diet for 12 wk. Specimens were stained with F4/80 for macrophages, or (d and e) CD3 for T cells, represented by the brown staining (arrows). The increase of macrophages and T cells is quantified in c and f, respectively. Macrophages and T cell content were significantly higher in the lesions of gld.apoE −/− mice (n = 11) compared with apoE −/− (n = 10) (*, P < 0.001 vs. apoE −/−). (g) Representative photomicrograph of TUNEL staining in lesions of apoE −/− mice. (h) Representative photomicrograph of TUNEL staining in lesions of gld-apoE −/− mice showing a greater amount of TUNEL-positive fragments than in the apoE −/− lesions. The (−)TUNEL condition indicates sections processed without the terminal deoxynucleotidyl transferase enzyme to assess background autofluorescence of the elastic lamina.
Figure 3.
Lipid profiles of apoE −/− and gld.apoE −/− mice maintained on normal diet and Western diet. (a) Total cholesterol levels were determined in gld.apoE −/− (n = 32), apoE −/− (n = 31), gld (n = 26), and wild type (n = 31) on Western diet and in gld.apoE −/− (n = 11), apoE −/− (n = 8), gld (n = 5), and wild type (n = 5) on normal diet (*, P < 0.001 vs. gld, wild type; †, P < 0.001 vs. apoE −/−). (b) Fast performance liquid chromatography profiles of sera from gld.apoE −/− and apoE −/− mice maintained on normal and Western diet. VLDL, LDL, and high density lipoprotein fraction locations are indicated.
Figure 4.
Lymphadenopathy and splenomegaly in gld and gld.apoE −/− mice. (a) Representative spleens excised from paired littermates of wild-type, gld, and gld.apoE −/− mice. (b) Representative submandibular lymph nodes (arrows) dissected from paired littermates of wild-type, gld, and gld.apoE −/− mice. Inset shows higher magnification image of lymph node from wild-type mouse. Diagram illustrates dissected area. (c) Spleen weights in the different strains of mice fed normal diet: gld.apoE −/− (n = 11), apoE −/− (n = 8), gld (n = 5), and wild type (n = 5); or Western diet: gld.apoE −/− (n = 26), apoE −/− (n = 31), gld (n = 26), and wild type (n = 31) (*, P < 0.001 vs. gld; †, P < 0.05 vs. gld; ‡, P < 0.001 vs. apoE −/−, wild type). (d) Lymph node weights in the different strains of mice fed normal diet: gld.apoE −/− (n = 11), apoE −/− (n = 8), gld (n = 5), and wild type (n = 5); or Western diet: gld.apoE −/− (n = 26), apoE −/− (n = 31), gld (n = 26), and wild type (n = 31) (*, P < 0.001 vs. gld; †, P < 0.001 vs. apoE −/−, wild type).
Figure 5.
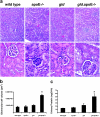
Evidence of renal dysfunction in the gld.apoE −/− mouse. (a) Representative hematoxylin and eosin stained sections of renal tissue showing typical glomeruli in gld.apoE −/− mice compared with wild-type, apoE −/−, and gld mice when maintained on Western diet. (top) Magnification, 100. (bottom) Magnification, 400. (b) Quantification of glomerular tuft volume revealed a statistically significant increase in glomerular size in the gld.apoE −/− mice compared with the control groups (*, P < 0.001). (c) The output of urinary protein by gld.apoE −/− mice maintained on normal diet was significantly increased compared with wild type, apoE −/−, and gld (*, P < 0.001).
Figure 6.
Elevated apoptotic fragments in lymph node and circulation of gld.apoE −/− mice. (a–c) Lymph node sections from animals fed Western diet were stained with TUNEL (FITC) and (d) quantified showing number of TUNEL-positive cells in the different strains of mice maintained on normal or Western diet (*, P < 0.05 vs. gld.apoE −/− on normal diet). gld.apoE −/− also showed a significant increase in TUNEL-positive cells compared with gld mice on normal or Western diet, respectively. Insets in a–c show higher magnification views. (e) FACS® analysis of TUNEL-positive cells in freshly isolated lymph node from Western diet–fed mice corroborates the histological findings (*, P < 0.05 vs. gld; P < 0.01 vs. WT, apoE −/−; †, P < 0.05 vs. WT, apoE −/−). (f) Platelet-free plasma isolated from different strains of mice on Western diet and assayed for apoptotic fragments by annexin V capture (*, P < 0.05 vs. gld; P < 0.01 vs. WT, apoE −/−).
Figure 7.
Evidence of impaired macrophage function in gld.apoE −/− mice. (a) Lymph node sections of wild-type mice stained with TUNEL (FITC) and F480 (PE) show colocalization of TUNEL-positive cells and macrophages (arrows, yellow merge). (b) Lymph node sections of gld.apoE −/− mice stained with TUNEL (FITC) and F480 (PE) show an abnormal morphology of macrophages and diminished colocalization. (c) Quantified results revealing a statistically significant decrease of macrophage ingestion of TUNEL-positive fragments in the gld and gld.apoE −/− mice, with gld.apoE −/− mice decreasing further than gld on both normal diet and Western diet (*, P < 0.05 vs. gld).
Figure 8.
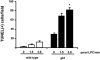
LPC promotes apoptotic body accumulation. LPC solutions or saline were infused for 120 min via tail vein in wild-type or gld mice. Lymph node sections were stained for TUNEL-positive cells. Quantified results reveal a significant increase in TUNEL-positive cells in gld mice after receiving LPC infusion (*, P < 0.001).
Similar articles
- Endothelial overexpression of Fas ligand decreases atherosclerosis in apolipoprotein E-deficient mice.
Yang J, Sato K, Aprahamian T, Brown NJ, Hutcheson J, Bialik A, Perlman H, Walsh K. Yang J, et al. Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1466-73. doi: 10.1161/01.ATV.0000134402.94963.2f. Epub 2004 Jun 3. Arterioscler Thromb Vasc Biol. 2004. PMID: 15178561 - Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice.
Zhou X, Paulsson G, Stemme S, Hansson GK. Zhou X, et al. J Clin Invest. 1998 Apr 15;101(8):1717-25. doi: 10.1172/JCI1216. J Clin Invest. 1998. PMID: 9541503 Free PMC article. - Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model.
Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, Walsh K. Aprahamian T, et al. J Immunol. 2006 Sep 1;177(5):3028-34. doi: 10.4049/jimmunol.177.5.3028. J Immunol. 2006. PMID: 16920939 Free PMC article. - Arterial inflammation and atherosclerosis.
Ludewig B, Zinkernagel RM, Hengartner H. Ludewig B, et al. Trends Cardiovasc Med. 2002 May;12(4):154-9. doi: 10.1016/s1050-1738(01)00166-9. Trends Cardiovasc Med. 2002. PMID: 12069754 Review. - The role of adaptive immunity in atherosclerosis.
Hansson GK, Zhou X, Törnquist E, Paulsson G. Hansson GK, et al. Ann N Y Acad Sci. 2000 May;902:53-62; discussion 62-4. doi: 10.1111/j.1749-6632.2000.tb06300.x. Ann N Y Acad Sci. 2000. PMID: 10865825 Review.
Cited by
- Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory.
Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Chang MK, et al. J Exp Med. 2004 Dec 6;200(11):1359-70. doi: 10.1084/jem.20031763. J Exp Med. 2004. PMID: 15583011 Free PMC article. - Macrophage death and defective inflammation resolution in atherosclerosis.
Tabas I. Tabas I. Nat Rev Immunol. 2010 Jan;10(1):36-46. doi: 10.1038/nri2675. Epub 2009 Dec 4. Nat Rev Immunol. 2010. PMID: 19960040 Free PMC article. Review. - ACAT inhibition reduces the progression of preexisting, advanced atherosclerotic mouse lesions without plaque or systemic toxicity.
Rong JX, Blachford C, Feig JE, Bander I, Mayne J, Kusunoki J, Miller C, Davis M, Wilson M, Dehn S, Thorp E, Tabas I, Taubman MB, Rudel LL, Fisher EA. Rong JX, et al. Arterioscler Thromb Vasc Biol. 2013 Jan;33(1):4-12. doi: 10.1161/ATVBAHA.112.252056. Epub 2012 Nov 8. Arterioscler Thromb Vasc Biol. 2013. PMID: 23139293 Free PMC article. - Systemic lupus erythematosus aggravates atherosclerosis by promoting IgG deposition and inflammatory cell imbalance.
Liu T, Shi N, Zhang S, Silverman GJ, Duan XW, Zhang S, Niu H. Liu T, et al. Lupus. 2020 Mar;29(3):273-282. doi: 10.1177/0961203320904779. Epub 2020 Feb 19. Lupus. 2020. PMID: 32075511 Free PMC article. - Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development.
Wilensky RL, Shi Y, Mohler ER 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Wilensky RL, et al. Nat Med. 2008 Oct;14(10):1059-66. doi: 10.1038/nm.1870. Epub 2008 Sep 21. Nat Med. 2008. PMID: 18806801 Free PMC article.
References
- Kotzin, B.L. 1996. Systemic lupus erythematosus. Cell. 85:303–306. - PubMed
- Savill, J., I. Dransfield, C. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975. - PubMed
- Potter, P.K., J. Cortes-Hernandez, P. Quartier, M. Botto, and M.J. Walport. 2003. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J. Immunol. 170:3223–3232. - PubMed
- Yasutomo, K., T. Horiuchi, S. Kagami, H. Tsukamoto, C. Hashimura, M. Urushihara, and Y. Kuroda. 2001. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 28:313–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017241/AG/NIA NIH HHS/United States
- AR40197/AR/NIAMS NIH HHS/United States
- R01 AG015052/AG/NIA NIH HHS/United States
- AG15052/AG/NIA NIH HHS/United States
- R37 AG015052/AG/NIA NIH HHS/United States
- R01 AR040197/AR/NIAMS NIH HHS/United States
- AG17241/AG/NIA NIH HHS/United States
- DK02597/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous